Bell Ringer Mole Mass Materials Needed 1 2

Bell Ringer
Mole- Mass

Materials Needed 1. 2. 3. 4. 5. 6. Periodic Table Interactive Notebook Guided Notes Pen/Pencil Enthusiasm Scientific Calculator…. ?
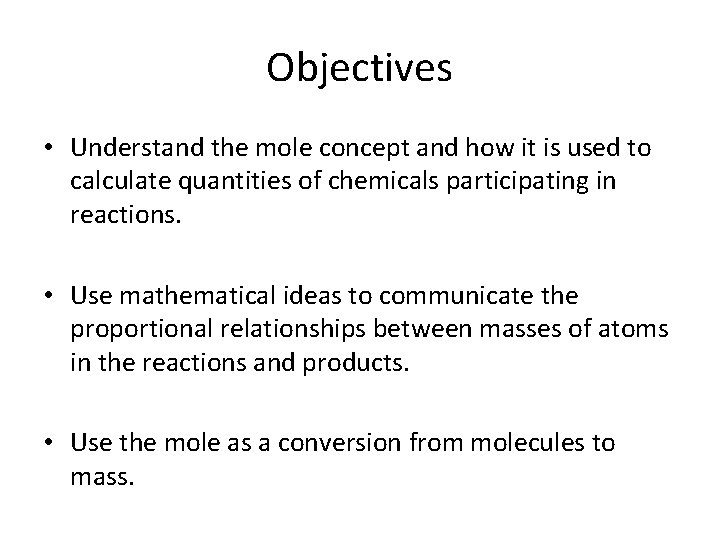
Objectives • Understand the mole concept and how it is used to calculate quantities of chemicals participating in reactions. • Use mathematical ideas to communicate the proportional relationships between masses of atoms in the reactions and products. • Use the mole as a conversion from molecules to mass.
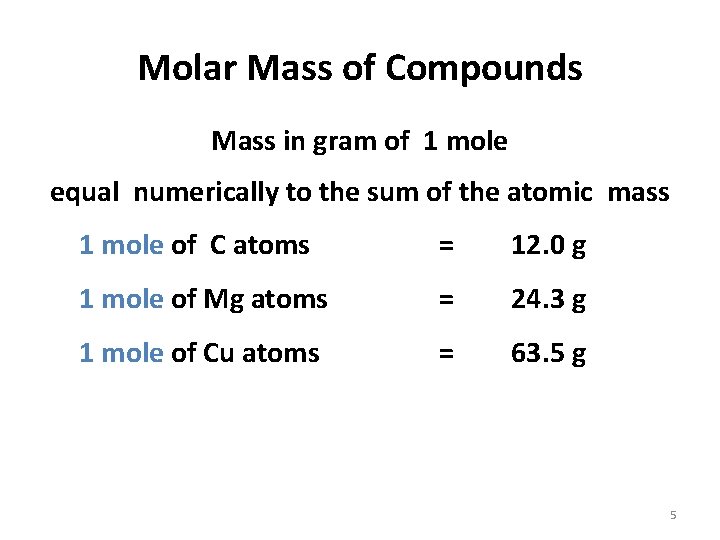
Molar Mass of Compounds Mass in gram of 1 mole equal numerically to the sum of the atomic mass 1 mole of C atoms = 12. 0 g 1 mole of Mg atoms = 24. 3 g 1 mole of Cu atoms = 63. 5 g 5
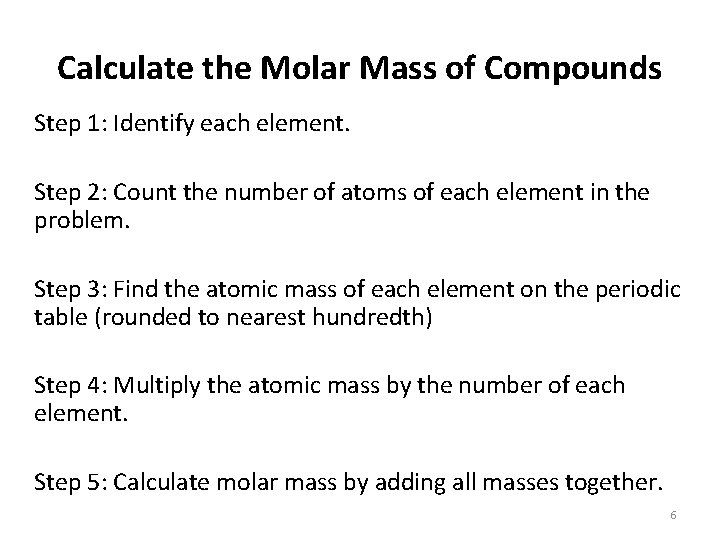
Calculate the Molar Mass of Compounds Step 1: Identify each element. Step 2: Count the number of atoms of each element in the problem. Step 3: Find the atomic mass of each element on the periodic table (rounded to nearest hundredth) Step 4: Multiply the atomic mass by the number of each element. Step 5: Calculate molar mass by adding all masses together. 6
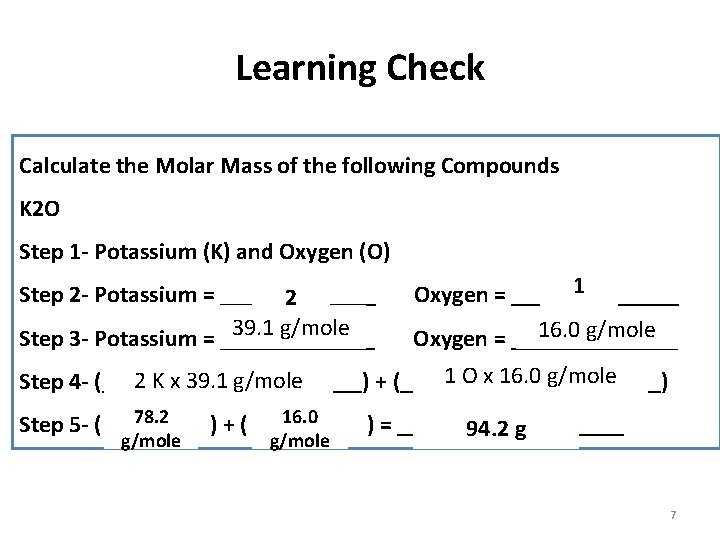
Learning Check Calculate the Molar Mass of the following Compounds K 2 O Step 1 - Potassium (K) and Oxygen (O) 1 Step 2 - Potassium = _______ Oxygen = _______ 2 39. 1 g/mole 16. 0 g/mole Step 3 - Potassium = _______ Oxygen = _______ 1 O x 16. 0 g/mole 2 K x 39. 1 g/mole Step 4 - (_____ x ______) + (_____ x _____) 78. 2 16. 0 Step 5 - ( ) + ( ) = __________ 94. 2 g g/mole 7
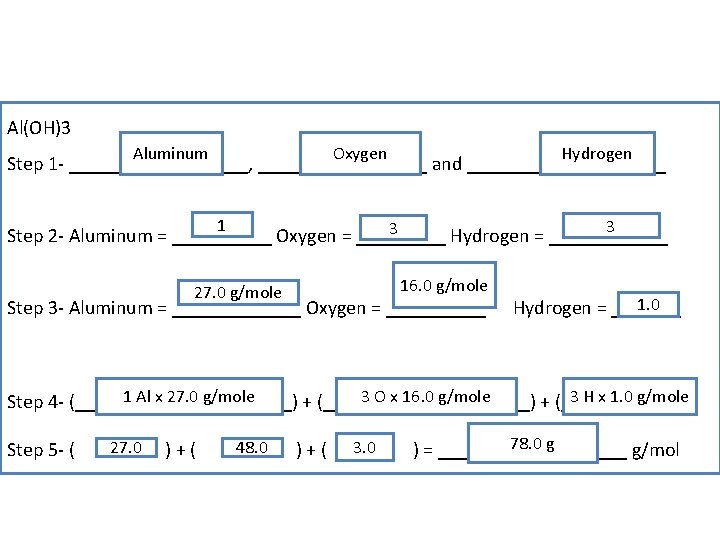
Al(OH)3 Aluminum Oxygen Hydrogen Step 1 - _________, _________ and __________ 1 3 3 Step 2 - Aluminum = _____ Oxygen = _____ Hydrogen = ______ 16. 0 g/mole 27. 0 g/mole Step 3 - Aluminum = _______ Oxygen = _____ 1. 0 Hydrogen = _______ 1 Al x 27. 0 g/mole 3 O x 16. 0 g/mole 3 H x 1. 0 g/mole Step 4 - (_____ x ______) + (_____ x _____) Step 5 - ( 27. 0 )+( 48. 0 )+( 3. 0 78. 0 g ) = __________ g/mol
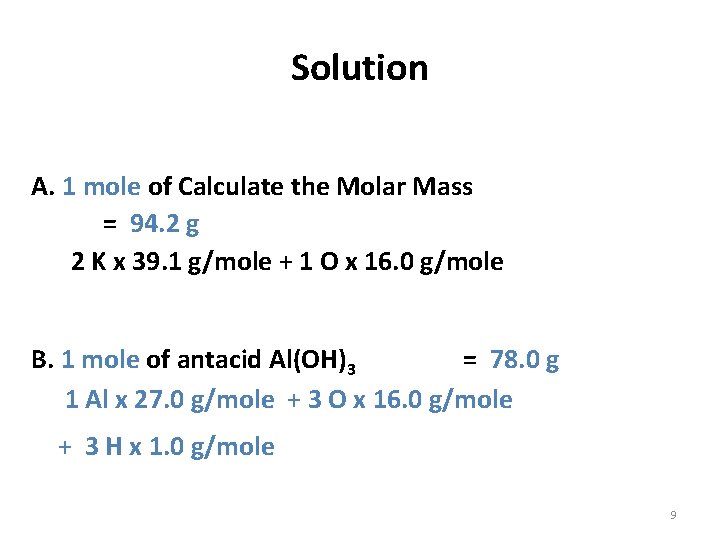
Solution A. 1 mole of Calculate the Molar Mass = 94. 2 g 2 K x 39. 1 g/mole + 1 O x 16. 0 g/mole B. 1 mole of antacid Al(OH)3 = 78. 0 g 1 Al x 27. 0 g/mole + 3 O x 16. 0 g/mole + 3 H x 1. 0 g/mole 9
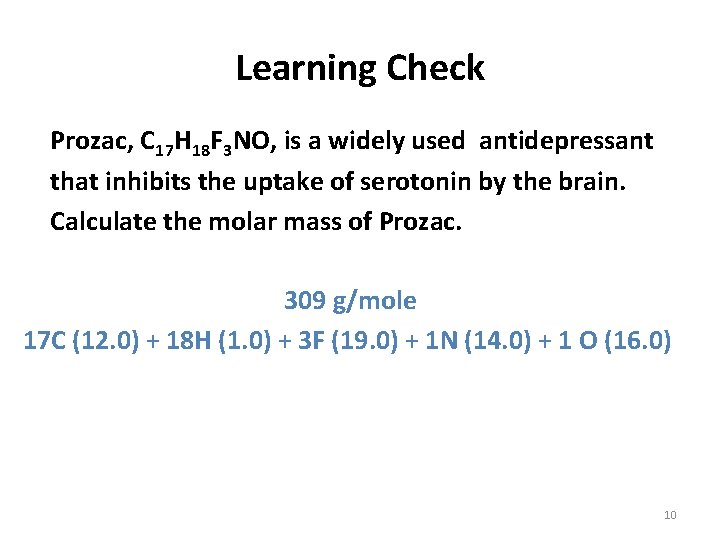
Learning Check Prozac, C 17 H 18 F 3 NO, is a widely used antidepressant that inhibits the uptake of serotonin by the brain. Calculate the molar mass of Prozac. 309 g/mole 17 C (12. 0) + 18 H (1. 0) + 3 F (19. 0) + 1 N (14. 0) + 1 O (16. 0) 10
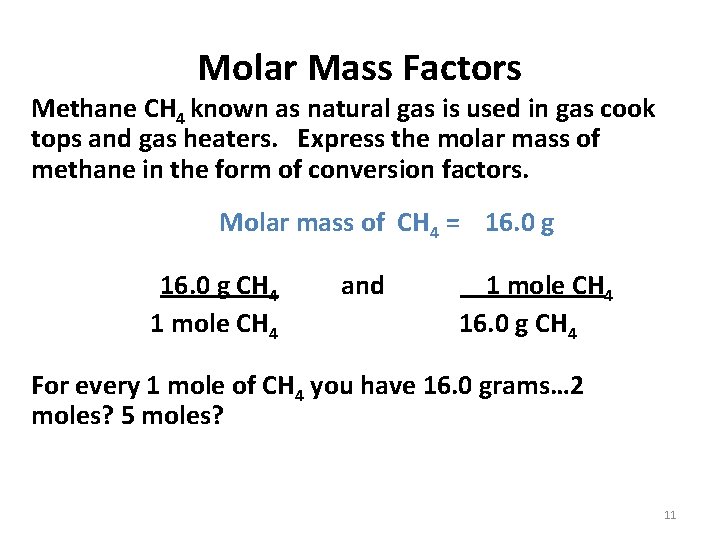
Molar Mass Factors Methane CH 4 known as natural gas is used in gas cook tops and gas heaters. Express the molar mass of methane in the form of conversion factors. Molar mass of CH 4 = 16. 0 g CH 4 and 1 mole CH 4 16. 0 g CH 4 For every 1 mole of CH 4 you have 16. 0 grams… 2 moles? 5 moles? 11
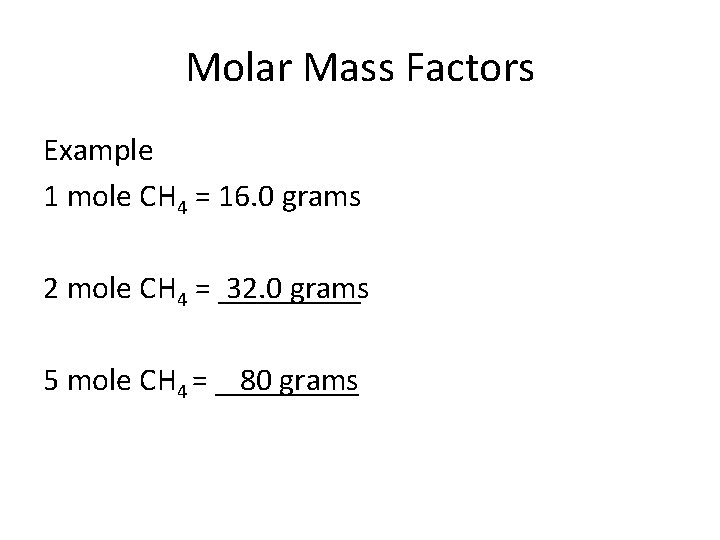
Molar Mass Factors Example 1 mole CH 4 = 16. 0 grams 2 mole CH 4 = _____ 32. 0 grams 80 grams 5 mole CH 4 = _____
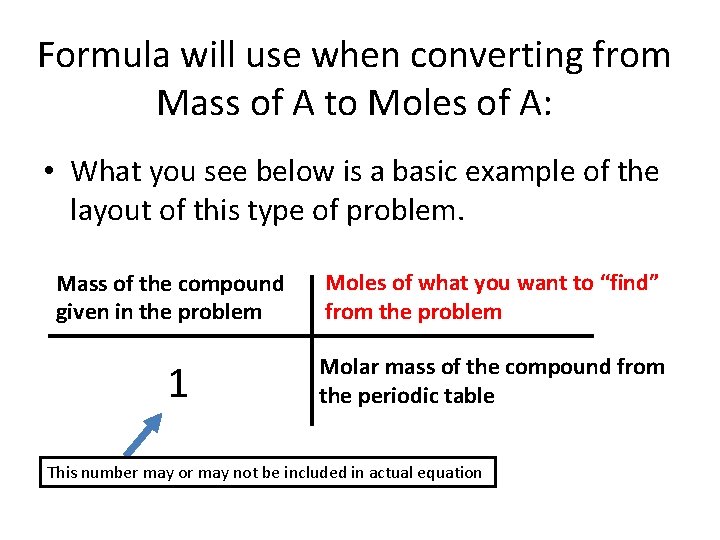
Formula will use when converting from Mass of A to Moles of A: • What you see below is a basic example of the layout of this type of problem. Mass of the compound given in the problem Moles of what you want to “find” from the problem 1 Molar mass of the compound from the periodic table This number may or may not be included in actual equation
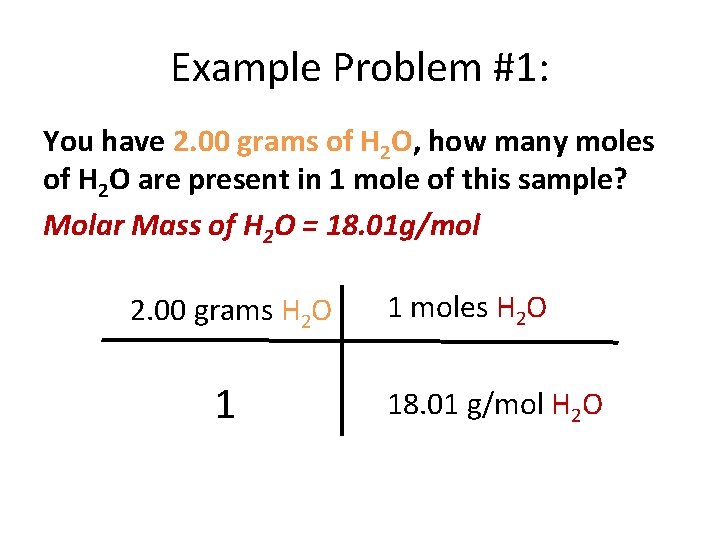
Example Problem #1: You have 2. 00 grams of H 2 O, how many moles of H 2 O are present in 1 mole of this sample? Molar Mass of H 2 O = 18. 01 g/mol 2. 00 grams H 2 O 1 1 moles H 2 O 18. 01 g/mol H 2 O
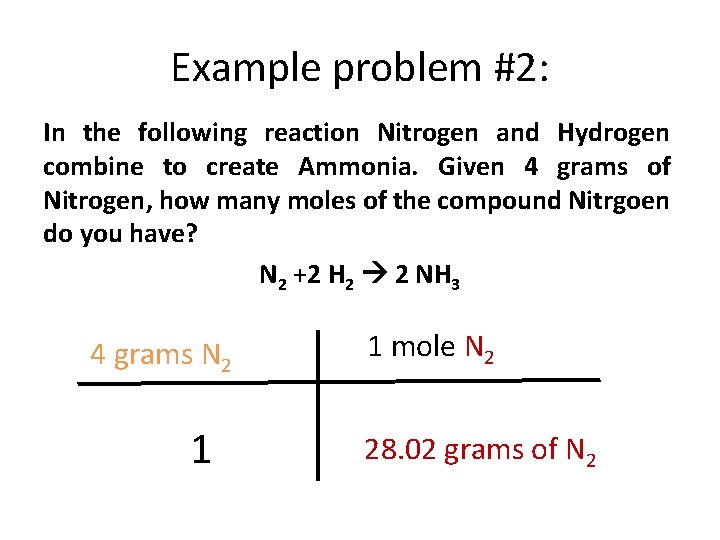
Example problem #2: In the following reaction Nitrogen and Hydrogen combine to create Ammonia. Given 4 grams of Nitrogen, how many moles of the compound Nitrgoen do you have? N 2 +2 H 2 2 NH 3 4 grams N 2 1 1 mole N 2 28. 02 grams of N 2
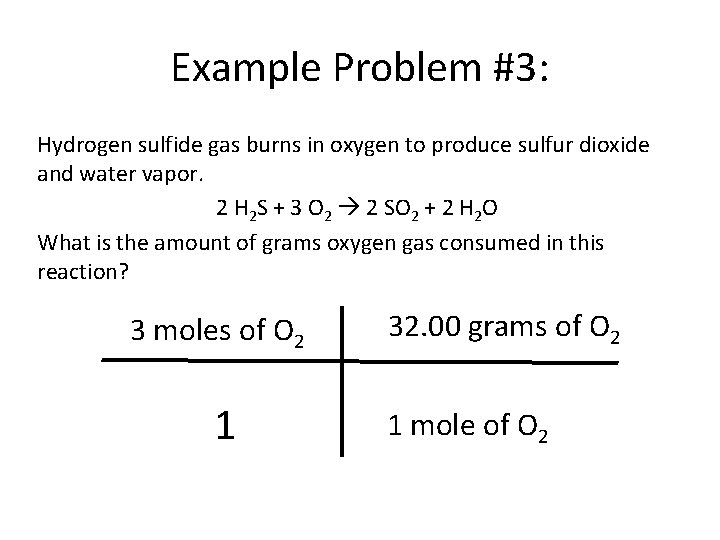
Example Problem #3: Hydrogen sulfide gas burns in oxygen to produce sulfur dioxide and water vapor. 2 H 2 S + 3 O 2 2 SO 2 + 2 H 2 O What is the amount of grams oxygen gas consumed in this reaction? 3 moles of O 2 1 32. 00 grams of O 2 1 mole of O 2
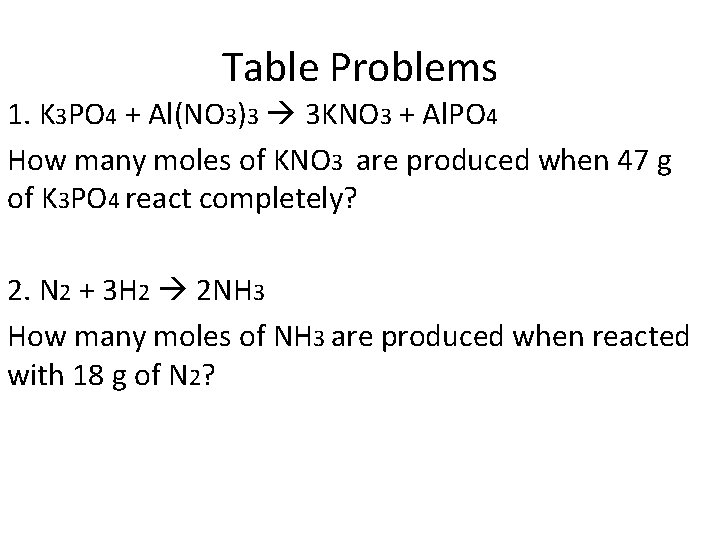
Table Problems 1. K 3 PO 4 + Al(NO 3)3 3 KNO 3 + Al. PO 4 How many moles of KNO 3 are produced when 47 g of K 3 PO 4 react completely? 2. N 2 + 3 H 2 2 NH 3 How many moles of NH 3 are produced when reacted with 18 g of N 2?

Independent Work Time Complete all of the problems on the Independent Practice Worksheet. There should be NO talking during this time Work on this BY YOURSELF.

Exit Slip 1. ____P + ____Br 2 ___PBr 3 If Alexis used 15 g Br 2 to make a reaction, calculate how many moles of PBr 3 she must have produced? 2. Given the following equation, If 0. 5 g of Al 2(SO 3)3 is reacted, determine how many moles of Al(OH)3 is produced. Al 2(SO 3)3 + 6 Na. OH 3 Na 2 SO 3 + 2 Al(OH)3

Independent Work Time Complete all of the problems on the Independent Practice Worksheet. There should be NO talking during this time Work on this BY YOURSELF.
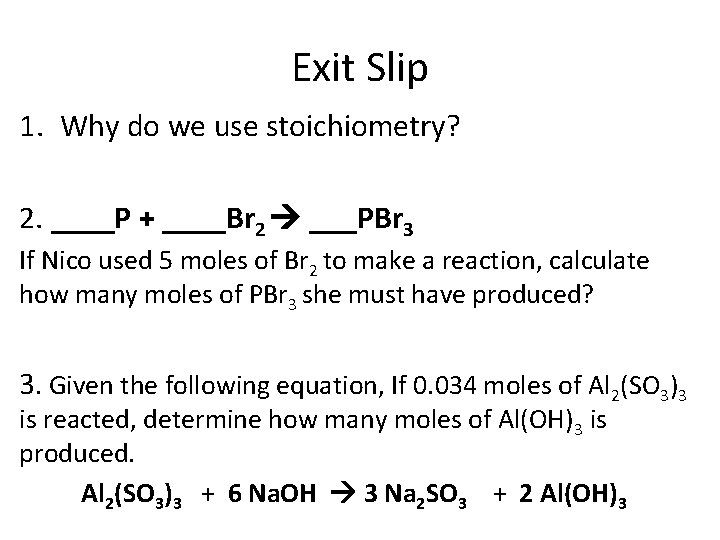
Exit Slip 1. Why do we use stoichiometry? 2. ____P + ____Br 2 ___PBr 3 If Nico used 5 moles of Br 2 to make a reaction, calculate how many moles of PBr 3 she must have produced? 3. Given the following equation, If 0. 034 moles of Al 2(SO 3)3 is reacted, determine how many moles of Al(OH)3 is produced. Al 2(SO 3)3 + 6 Na. OH 3 Na 2 SO 3 + 2 Al(OH)3
- Slides: 21