The Mole Chemical Formulas The Mole n Mole











- Slides: 17

The Mole & Chemical Formulas

The Mole n Mole, abbreviated mol, is the SI (metric) base unit to measure the amount of a substance – It is called Avogadro’s number in honor of Italian physicist Amedeo Avogadro, who discovered one mole of gas in 1811 – 6. 0221367 x 1023 representative particles 602, 000, 000, 000

Use of the Mole n One wouldn’t use this unit for everything – A mole of marbles would cover the surface of the Earth down to approximately 6 kilometers – Although a mole of $1. 00 bills would be nice! n It is used to measure the amount of molecules in a substance

Conversion Recall n 5, 280 feet = 1 mile n How many miles would you have in 12, 369 feet? n It is the same system of proportions except now its… – 1 mole = 6. 02 x 1023 – So, if you had 2. 5 moles of H 2 O, how many atoms of water do you have?

Recall n Determine the number of atoms in 2. 75 moles of Zn? n If you had 9 moles of Cu(OH), how many molecules are present? n Lets change it up! – How many moles of H 2 SO 4 if you had 2. 11 x 1024 molecules?

Mass & The Moles n A dozen oranges won’t have the same mass a dozen watermelons – But you do have exactly the same amount of each § 12 n Since they have different compositions, their mass will be different as well – Thus, the mass of 1 mole of silver nitrate (Ag. NO 3) will be different than 1 mole of oxygen gas (O 2)

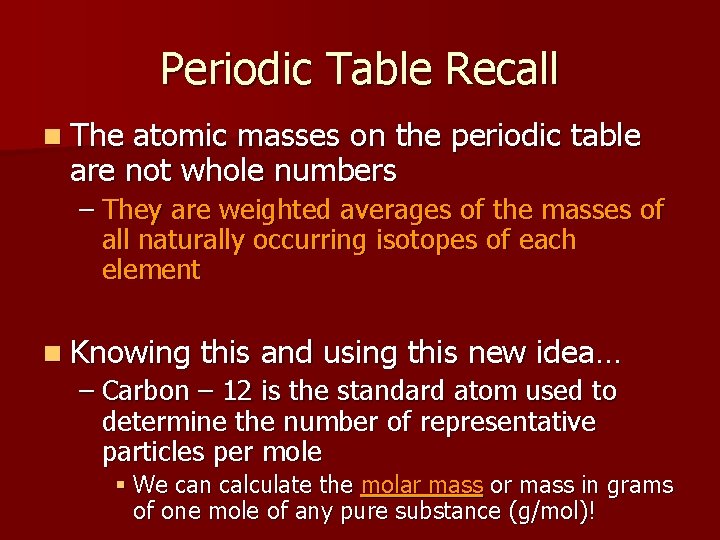
Periodic Table Recall n The atomic masses on the periodic table are not whole numbers – They are weighted averages of the masses of all naturally occurring isotopes of each element n Knowing this and using this new idea… – Carbon – 12 is the standard atom used to determine the number of representative particles per mole § We can calculate the molar mass or mass in grams of one mole of any pure substance (g/mol)!

So What Does This Mean? n Periodic Table Transitions** – Carbon has a mass of 12 § Thus 12 g of carbon = 1 mole of Carbon – Manganese (Mn) has an atomic mass of 54. 94 amu § 55 g of Mn = 1 mol Mn n n You Try! – – – 3 mol Mn = ? g Mn 136 g Pb = ? Mol Pb Challenge* § How many grams of gold are found in 7. 65 x 1022 Au atoms? This works for compounds as well as single elements – 1. 00 g H 2 O = ? Mol H 2 O – 1. 00 g H 2 O = ? Molecules H 2 O

Challenge n Freon – CCl 2 F 2 – This is a molecule whose formula is known – In each molecule, there is 1 atom of Carbon, 2 atoms of Chlorine and 2 atoms of Fluorine – If one were asked, how many moles of F are there in 5. 50 moles of Freon, what would you do? 5. 50 mol Freon * 2 mol F atoms = 11 mol F atoms 1 1 mol Freon

Calculate the following: 1. How many molecules are found in 1. 8 moles of water? 2. How many moles are found in 2. 51 x 1025 molecules of sodium chloride? 3. How many grams would be on the balance if one obtained 4. 94 x 1024 molecules of ammonium nitrate? (Need the formula for the compound!) 4. What if you measured 1. 67 grams of pure silver, how many atoms would that be?

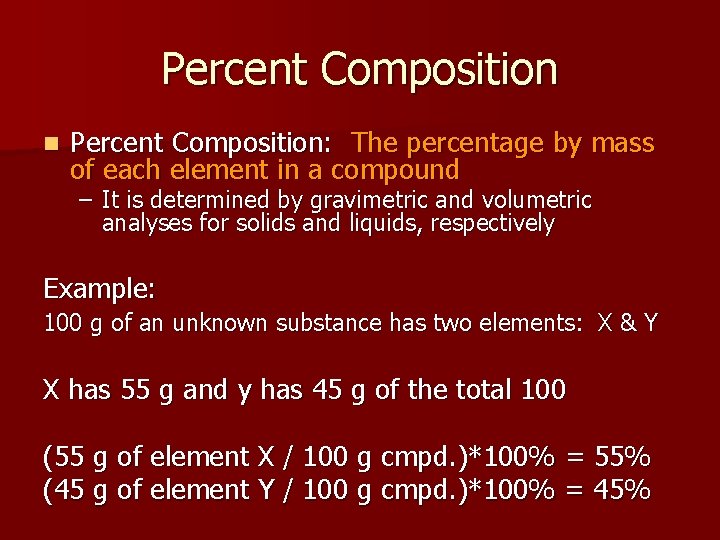
Percent Composition n Percent Composition: The percentage by mass of each element in a compound – It is determined by gravimetric and volumetric analyses for solids and liquids, respectively Example: 100 g of an unknown substance has two elements: X & Y X has 55 g and y has 45 g of the total 100 (55 g of element X / 100 g cmpd. )*100% = 55% (45 g of element Y / 100 g cmpd. )*100% = 45%

Percent Composition n If you know the compound in question, you can calculate the percent composition for the known atoms Example: 18. 02 g/mol H 2 O Hydrogen (H) – 1. 00794 g Oxygen (O) – 15. 9994 g 1. 00794 g * 2 Hydrogen in water = 2. 02 g (2. 02 g H / 18. 02 g H 2 O) * 100 = 11. 2 % H 16. 00 g O * 1 Oxygen in water = 16. 00 g (16. 00 g O / 18. 02 g H 2 O) * 100 = 88. 8 % O So, water is 11. 2% Hydrogen and 88. 8% Oxygen Try 8. 00 g of Cu(OH)

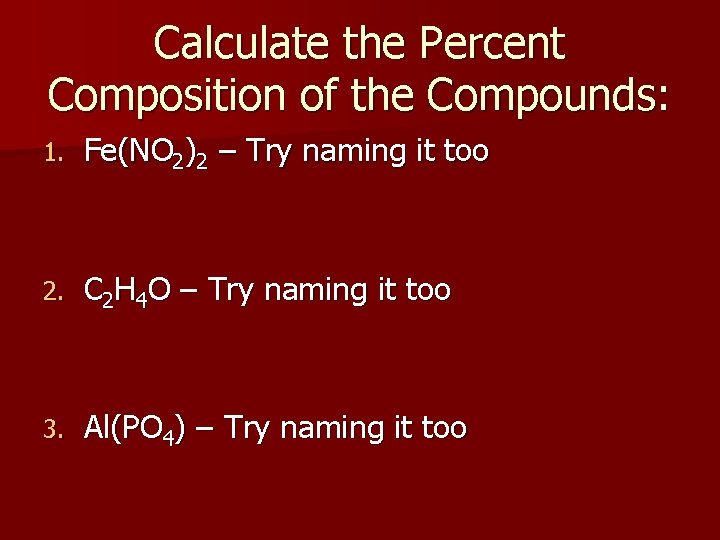
Calculate the Percent Composition of the Compounds: 1. Fe(NO 2)2 – Try naming it too 2. C 2 H 4 O – Try naming it too 3. Al(PO 4) – Try naming it too

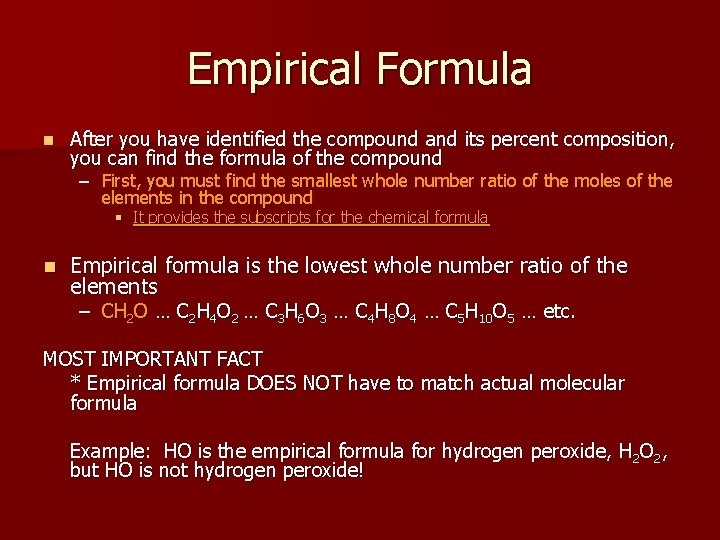
Empirical Formula n After you have identified the compound and its percent composition, you can find the formula of the compound – First, you must find the smallest whole number ratio of the moles of the elements in the compound § It provides the subscripts for the chemical formula n Empirical formula is the lowest whole number ratio of the elements – CH 2 O … C 2 H 4 O 2 … C 3 H 6 O 3 … C 4 H 8 O 4 … C 5 H 10 O 5 … etc. MOST IMPORTANT FACT * Empirical formula DOES NOT have to match actual molecular formula Example: HO is the empirical formula for hydrogen peroxide, H 2 O 2, but HO is not hydrogen peroxide!

Empirical Formula n You can assume the mass of the compound is 100 g if they give you percent composition. So if 40. 05 % is Sulfur (S) and 59. 95% is Oxygen (O), then there is 40. 05 g S and 59. 95 g. O Then simply convert to moles: 40. 05 g S * 1 mol S = 1. 249 mol S 1 32. 07 g S 59. 95 g O * 1 mol O 1 16 g O = 3. 747 mol O So the molar ratio is 1. 249: 3. 747 for S: O atoms This will produce integers but we want whole numbers How do we simplify? Take the smaller number and divide it and everything else by that number. 1. 249/1. 249 = 1 3. 747/1. 249 = 3 1: 3 Ratio so for every one S, there is three O. SO 3

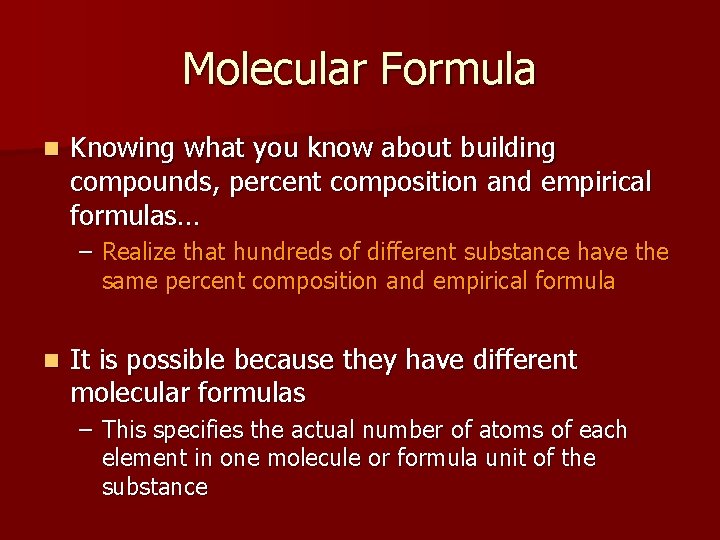
Molecular Formula n Knowing what you know about building compounds, percent composition and empirical formulas… – Realize that hundreds of different substance have the same percent composition and empirical formula n It is possible because they have different molecular formulas – This specifies the actual number of atoms of each element in one molecule or formula unit of the substance

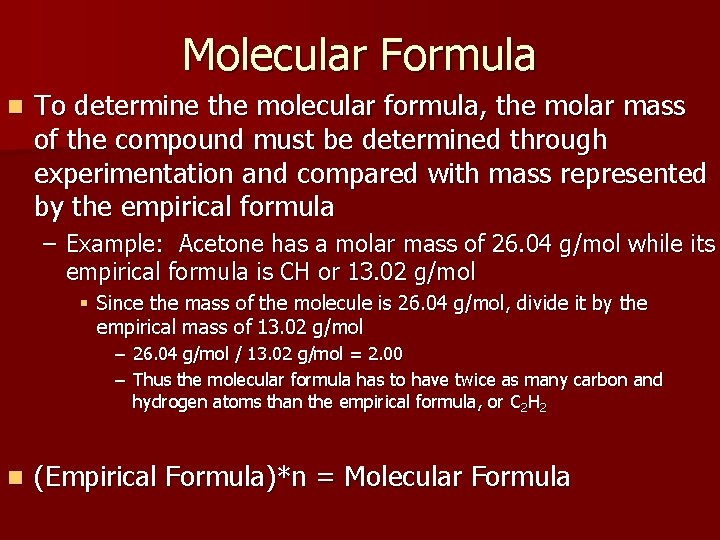
Molecular Formula n To determine the molecular formula, the molar mass of the compound must be determined through experimentation and compared with mass represented by the empirical formula – Example: Acetone has a molar mass of 26. 04 g/mol while its empirical formula is CH or 13. 02 g/mol § Since the mass of the molecule is 26. 04 g/mol, divide it by the empirical mass of 13. 02 g/mol – 26. 04 g/mol / 13. 02 g/mol = 2. 00 – Thus the molecular formula has to have twice as many carbon and hydrogen atoms than the empirical formula, or C 2 H 2 n (Empirical Formula)*n = Molecular Formula
 Modern chemistry chapter 7 review answers
Modern chemistry chapter 7 review answers Empirical formula and molecular formula pogil
Empirical formula and molecular formula pogil Mole formual
Mole formual Vapour density formula
Vapour density formula Gram to gram conversion
Gram to gram conversion Stoichiometry mole-mole
Stoichiometry mole-mole Mole mole factor
Mole mole factor Mass and grams
Mass and grams Stoichiometry worksheet #2 (mole-mass mass-mole problems)
Stoichiometry worksheet #2 (mole-mass mass-mole problems) Mole mass and mole volume relationships
Mole mass and mole volume relationships Molar mass of sucrose
Molar mass of sucrose Writing and naming chemical formulas
Writing and naming chemical formulas Chemical formulas list
Chemical formulas list Counting atoms worksheet
Counting atoms worksheet Hg3p compound name
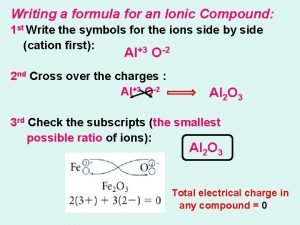
Hg3p compound name Writing the formula for ionic compounds
Writing the formula for ionic compounds How to write the chemical formula for ionic compounds
How to write the chemical formula for ionic compounds Chemical formulas
Chemical formulas