Unit 11 Stoichiometry MOLE RATIOS MOLE TO MOLE






- Slides: 13

Unit 11: Stoichiometry MOLE RATIOS MOLE TO MOLE CONVERSIONS

Irrelevant Information to Take Your Mind Off of Stoichiometry The first person to use the word “stoichiometry” was Nicephorus I, the ecumenical Patriarch of Constantinople in the early 9 th century. However, the term as he used it refers to the number of lines of text in the New Testament. This suggests that the term “stoichiometry” has been annoying people for over a millennium. Image: Classical Numismatic

Some stoich fun: Stoichiometry

What is stoichiometry? The quantitative relationships between the amounts of reactants used and the amounts of products formed by a chemical reaction. Which is just a fancy way of saying: ◦ “the method you use to figure out how much of a chemical you can make, or how much you need to produce a substance, during a reaction. ”

In order to do Stoich: 1. Know how to balance chemical equations 2. Know how to convert grams to moles/moles to grams 3. How to use mole ratios Can you do 1 and 2?

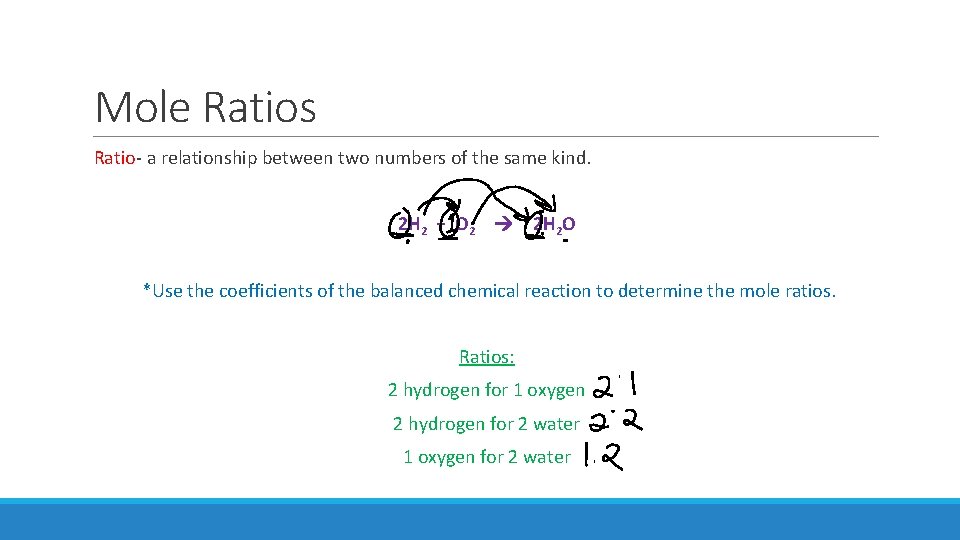
Mole Ratios Ratio- a relationship between two numbers of the same kind. 2 H 2 + O 2 2 H 2 O *Use the coefficients of the balanced chemical reaction to determine the mole ratios. Ratios: 2 hydrogen for 1 oxygen 2 hydrogen for 2 water 1 oxygen for 2 water

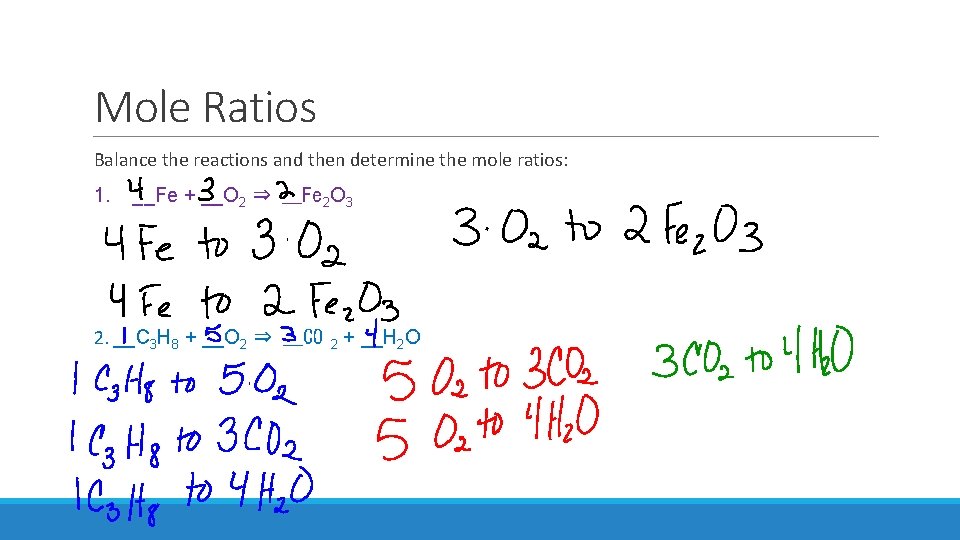
Mole Ratios Balance the reactions and then determine the mole ratios: 1. __Fe + __O 2 ⇒ __Fe 2 O 3 2. __C 3 H 8 + __O 2 ⇒ __CO 2 + __H 2 O

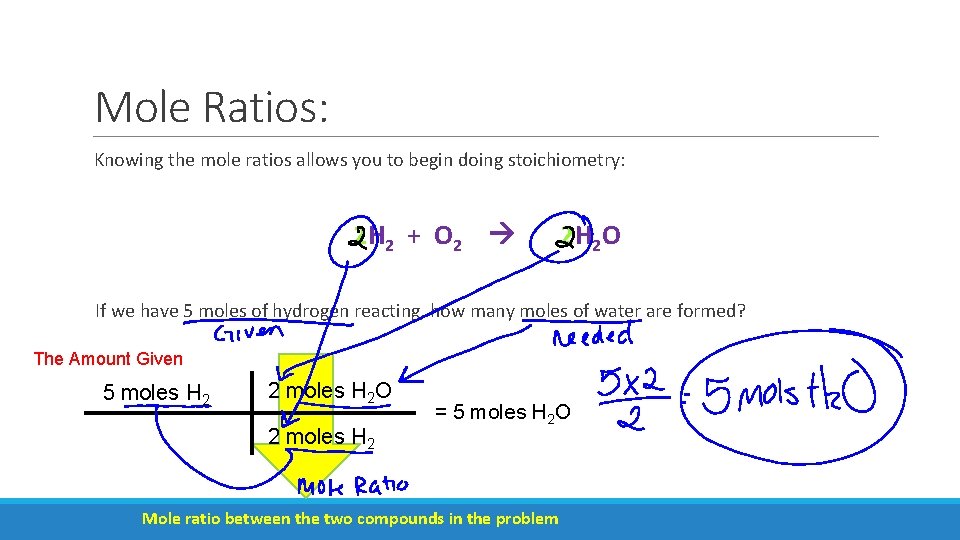
Mole Ratios: Knowing the mole ratios allows you to begin doing stoichiometry: 2 H 2 + O 2 2 H 2 O If we have 5 moles of hydrogen reacting, how many moles of water are formed? The Amount Given 5 moles H 2 2 moles H 2 O 2 moles H 2 = 5 moles H 2 O Mole ratio between the two compounds in the problem

Mole to Mole Conversions: We can determine the number of moles needed to produce, or how much of a substance will be produced from the number of moles we have to start with.

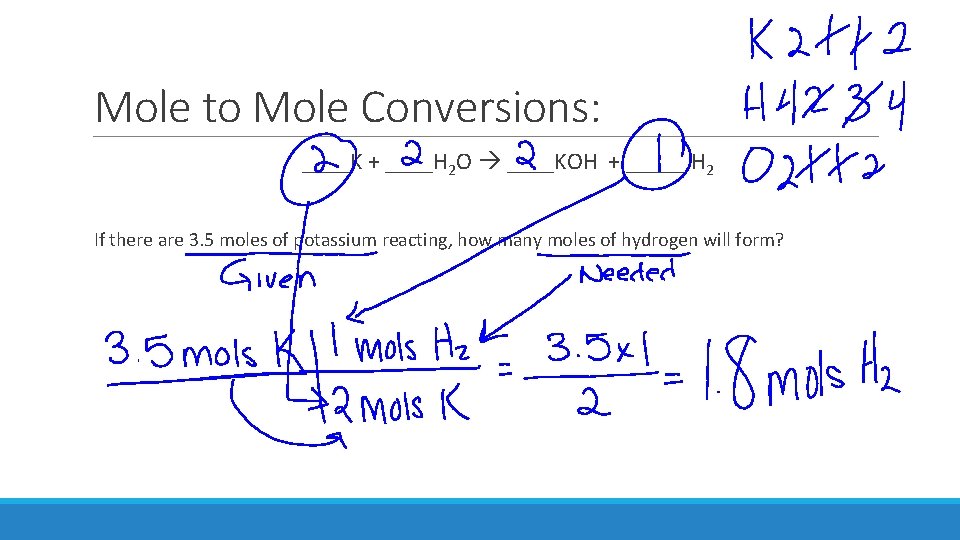
Mole to Mole Conversions: ____K + ____H 2 O ____KOH + _____ H 2 If there are 3. 5 moles of potassium reacting, how many moles of hydrogen will form?

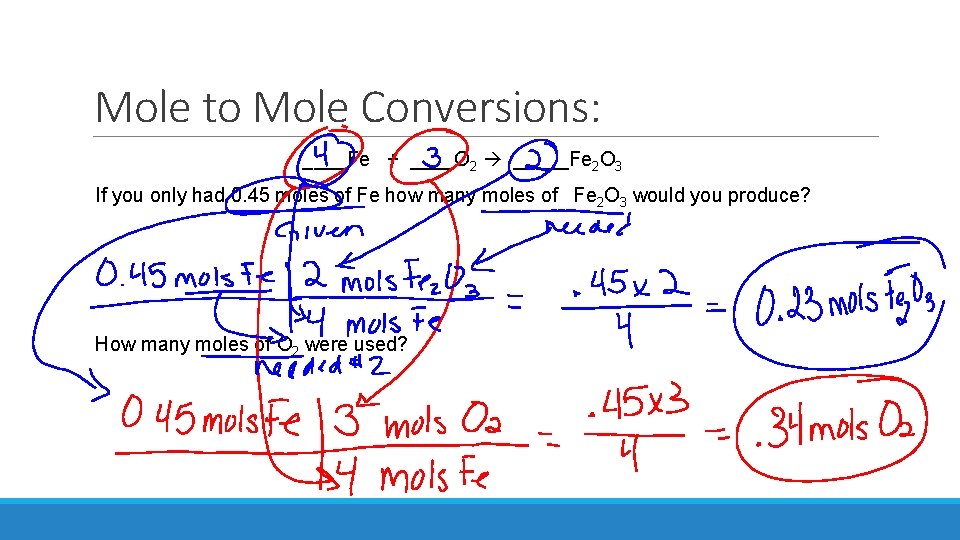
Mole to Mole Conversions: ____Fe + ____O 2 _____Fe 2 O 3 If you only had 0. 45 moles of Fe how many moles of Fe 2 O 3 would you produce? How many moles of O 2 were used?

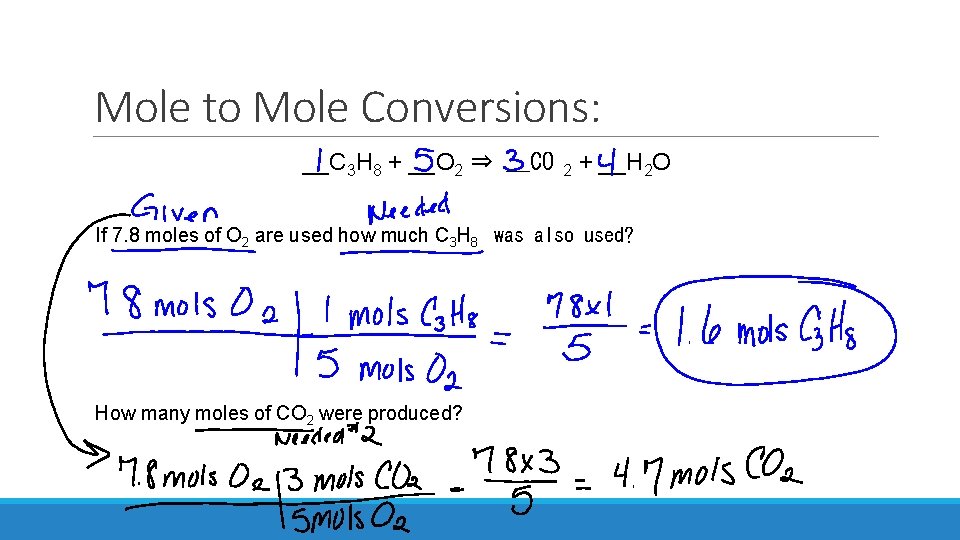
Mole to Mole Conversions: __C 3 H 8 + __O 2 ⇒ __CO 2 + __H 2 O If 7. 8 moles of O 2 are used how much C 3 H 8 was also used? How many moles of CO 2 were produced?

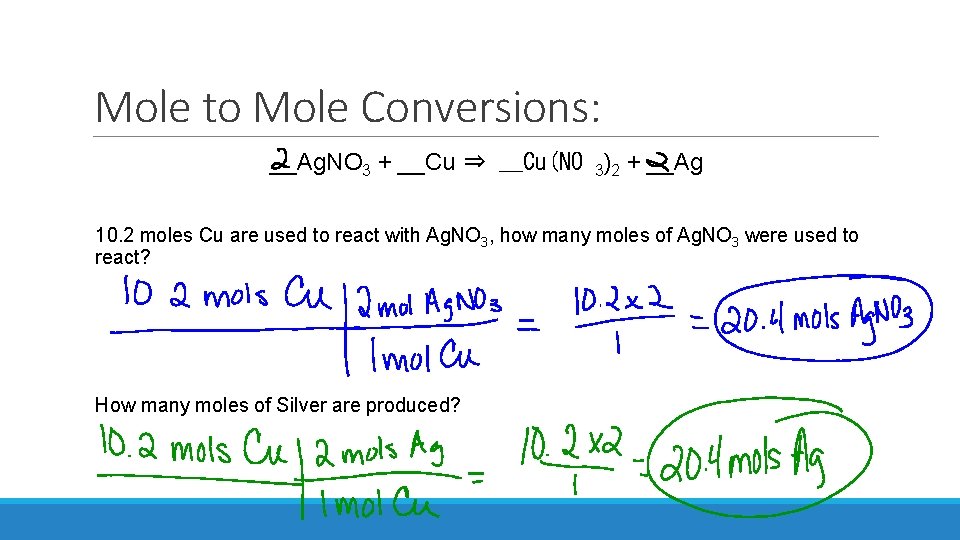
Mole to Mole Conversions: __Ag. NO 3 + __Cu ⇒ __Cu(NO 3)2 + __Ag 10. 2 moles Cu are used to react with Ag. NO 3, how many moles of Ag. NO 3 were used to react? How many moles of Silver are produced?