Stoichiometry Moles and Molar mass How Big is
Stoichiometry Moles and Molar mass

How Big is a Mole? One mole of marbles would cover the entire Earth (oceans included) for a depth of two miles. One mole of $1 bills stacked one on top of another would reach from the Sun to Pluto and back 7. 5 million times. It would take light 9500 years to travel from the bottom to the top of a stack of 1 mole of $1 bills.

Amedeo Avogadro ? quadrillions trillions billions thousands millions 1 mole = 60221367360000000 or 6. 022 x 1023

Welcome to Mole Island 1 mol = molar mass 1 mole = 22. 4 L @ STP 1 mol = 6. 02 x 1023 particles
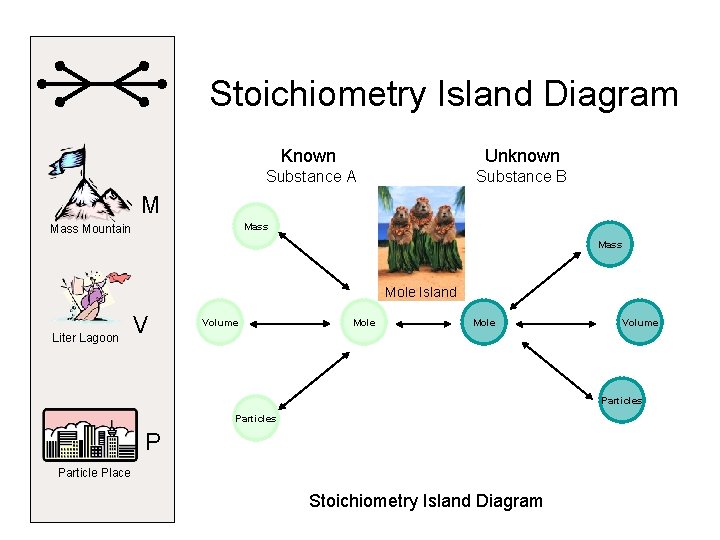
Stoichiometry Island Diagram Known Unknown Substance A Substance B M Mass Mountain Mass Mole Island Liter Lagoon V Volume Mole Volume Particles P Particle Place Stoichiometry Island Diagram
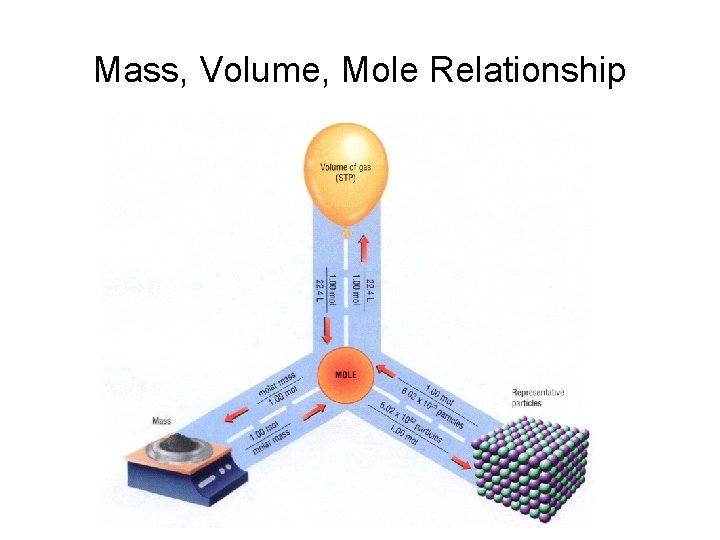
Mass, Volume, Mole Relationship
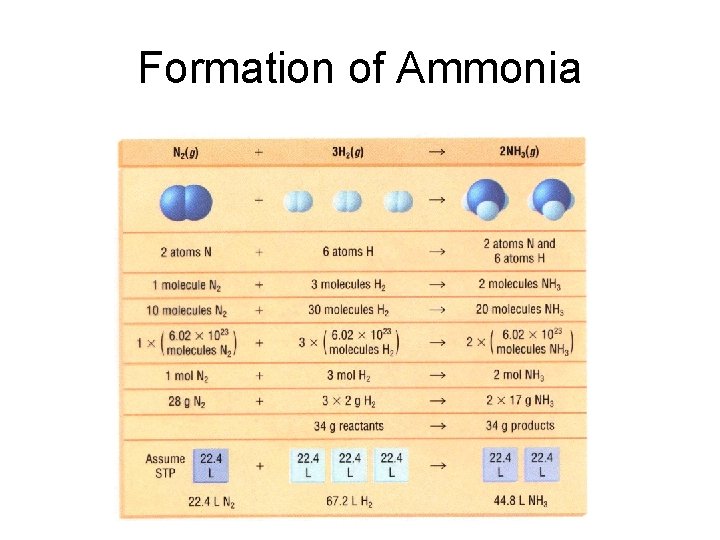
Formation of Ammonia

Example: What is the number of moles of 14 g Li? (Li: 7) Example: What is the mass of 0. 1 mole Ca? (Ca: 40) Example: Calculate the mole number of each of the followings. a) 13. 7 g Ba (Ba: 137) b) b) 10. 4 g Cr (Cr: 52) c) c) 635 g Cu (Cu: 63. 5) d) d) 12 g Mg (Mg: 24) Example: Calculate the mass of each of the following. a) 0. 1 mole Na (Na: 23) b) b) 0. 5 mole Be (Be: 9) c) c) 2 mole K (K: 39) d) d) 1. 5 mole Fe (Fe: 56)
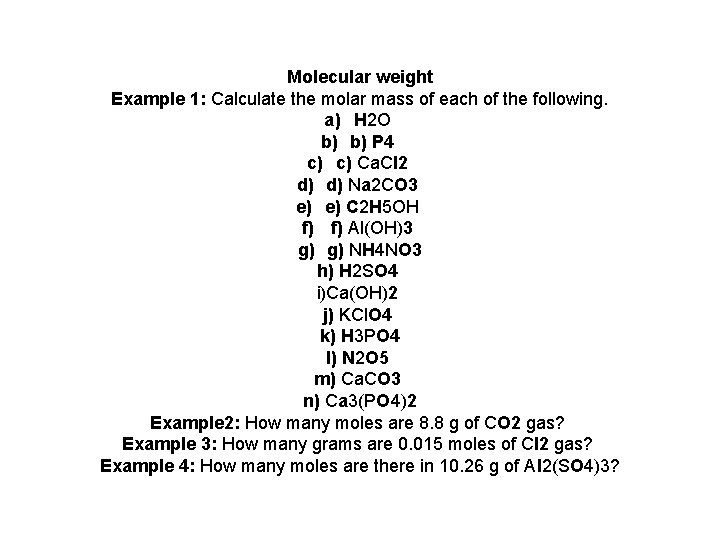
Molecular weight Example 1: Calculate the molar mass of each of the following. a) H 2 O b) b) P 4 c) c) Ca. Cl 2 d) d) Na 2 CO 3 e) e) C 2 H 5 OH f) f) Al(OH)3 g) g) NH 4 NO 3 h) H 2 SO 4 i)Ca(OH)2 j) KCl. O 4 k) H 3 PO 4 l) N 2 O 5 m) Ca. CO 3 n) Ca 3(PO 4)2 Example 2: How many moles are 8. 8 g of CO 2 gas? Example 3: How many grams are 0. 015 moles of Cl 2 gas? Example 4: How many moles are there in 10. 26 g of Al 2(SO 4)3?

1)Calculate the molar mass of Carbon dioxide 2) How many moles are there in 62. 5 grams of Na. HCO 3 3) Calculate the molar mass of nitrogen trioxide 4) Calculate the molar mass of acetic acid CH 3 COOH

5) Calculate the number of moles of nitrogen trioxide in 67. 8 grams 6) Calculate the number of grams in 2. 5 moles of acetic acid 7)Calculate the number of moles in 36. 2 g of acetic acid

Real life Stoichiometry
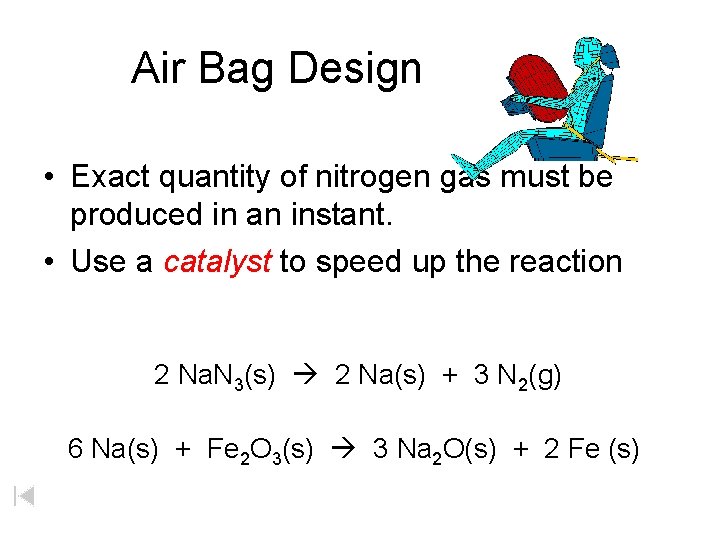
Air Bag Design • Exact quantity of nitrogen gas must be produced in an instant. • Use a catalyst to speed up the reaction 2 Na. N 3(s) 2 Na(s) + 3 N 2(g) 6 Na(s) + Fe 2 O 3(s) 3 Na 2 O(s) + 2 Fe (s)
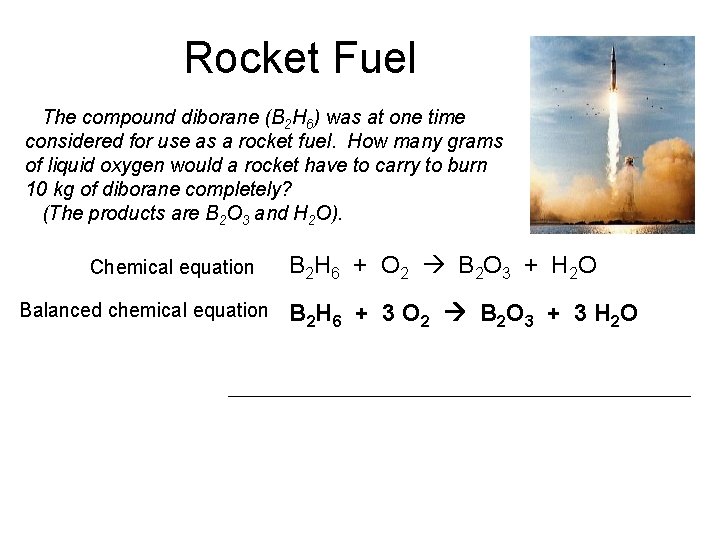
Rocket Fuel The compound diborane (B 2 H 6) was at one time considered for use as a rocket fuel. How many grams of liquid oxygen would a rocket have to carry to burn 10 kg of diborane completely? (The products are B 2 O 3 and H 2 O). Chemical equation Balanced chemical equation B 2 H 6 + O 2 B 2 O 3 + H 2 O B 2 H 6 + 3 O 2 B 2 O 3 + 3 H 2 O
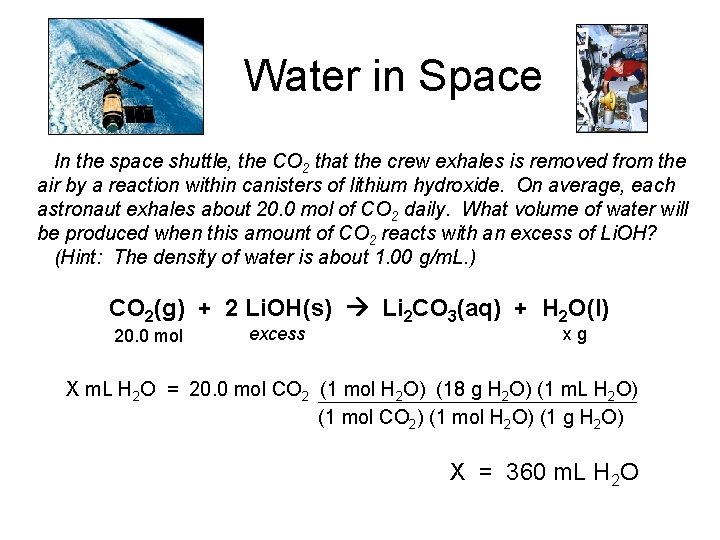
Water in Space In the space shuttle, the CO 2 that the crew exhales is removed from the air by a reaction within canisters of lithium hydroxide. On average, each astronaut exhales about 20. 0 mol of CO 2 daily. What volume of water will be produced when this amount of CO 2 reacts with an excess of Li. OH? (Hint: The density of water is about 1. 00 g/m. L. ) CO 2(g) + 2 Li. OH(s) Li 2 CO 3(aq) + H 2 O(l) 20. 0 mol excess xg X m. L H 2 O = 20. 0 mol CO 2 (1 mol H 2 O) (18 g H 2 O) (1 m. L H 2 O) (1 mol CO 2) (1 mol H 2 O) (1 g H 2 O) X = 360 m. L H 2 O
- Slides: 15