Molar Mass Conversions The Mole mole mol SI



- Slides: 18

Molar Mass & Conversions

The Mole mole (mol)- SI Unit for the amount of a substance that contains as many particles as there atoms in exactly 12 g of carbon-12. • A unit of counting, like the dozen.

Avogadro’s Number - the number of particles in exactly one mole of a pure substance. 1 mole = 6. 0221415 X 1023 1 mol = 6. 022 x 1023 Amedeo Avogadro

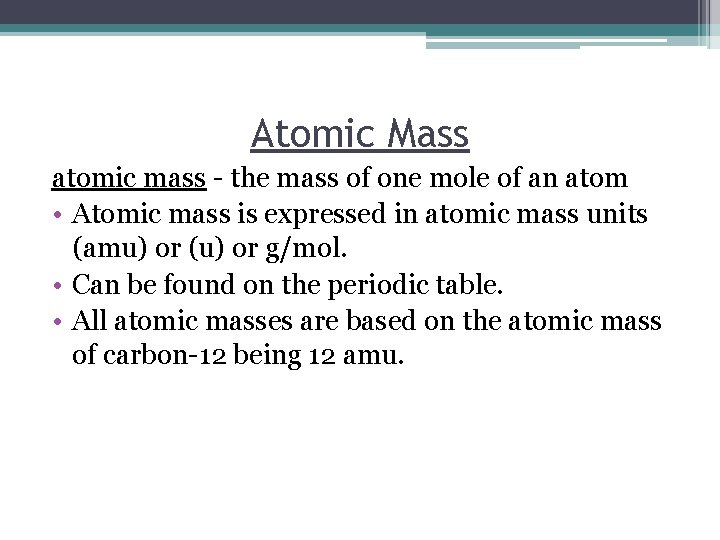
Atomic Mass atomic mass - the mass of one mole of an atom • Atomic mass is expressed in atomic mass units (amu) or (u) or g/mol. • Can be found on the periodic table. • All atomic masses are based on the atomic mass of carbon-12 being 12 amu.

Molar Mass molar mass - the mass of one mole of a pure substance. • Molar mass is written in units of amu or g/mol.

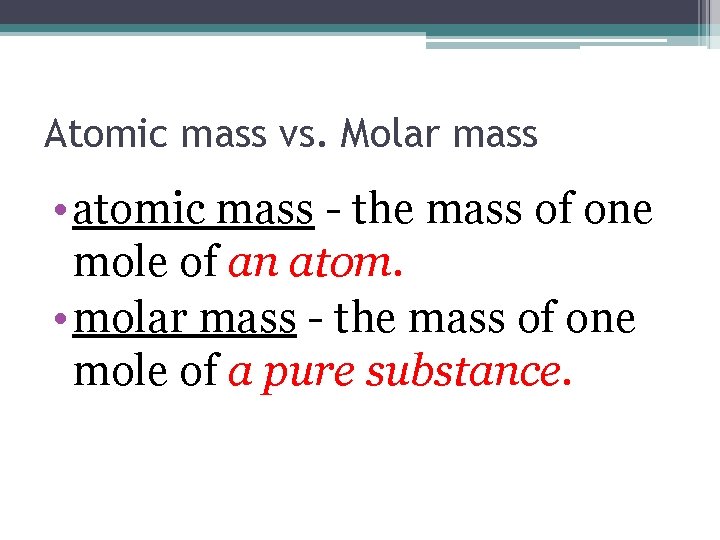
Atomic mass vs. Molar mass • atomic mass - the mass of one mole of an atom. • molar mass - the mass of one mole of a pure substance.

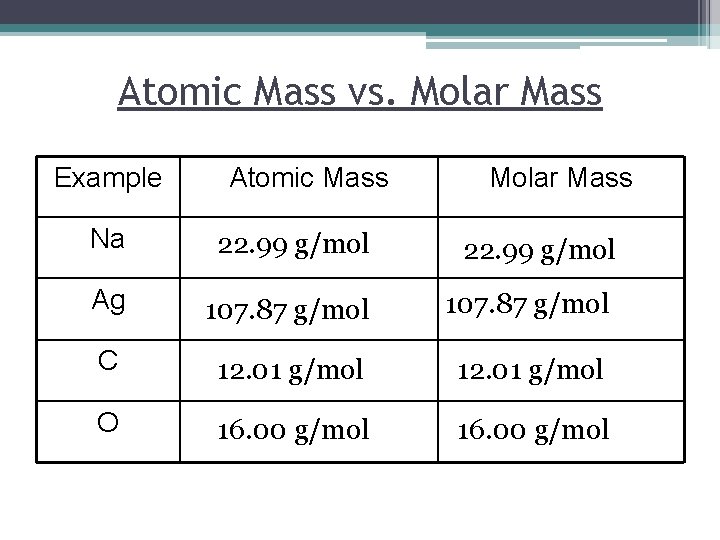
Atomic Mass vs. Molar Mass Example Atomic Mass Molar Mass Na 22. 99 g/mol Ag 107. 87 g/mol C 12. 01 g/mol O 16. 00 g/mol

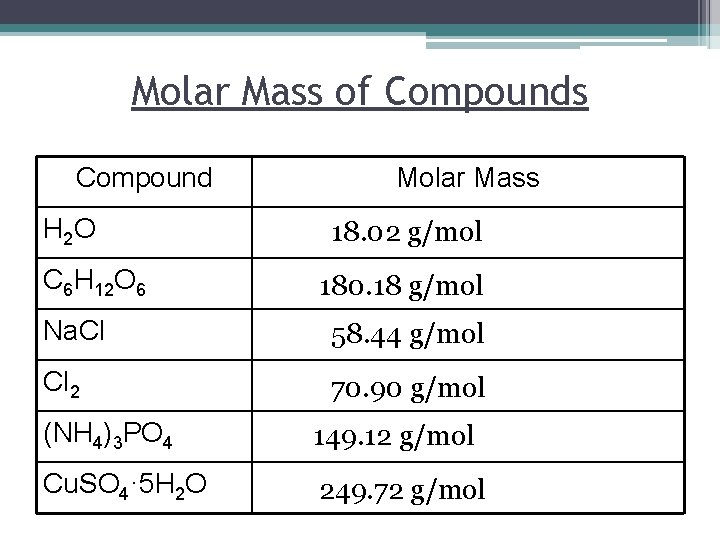
Molar Mass of Compounds Compound H 2 O C 6 H 12 O 6 Molar Mass 18. 02 g/mol 180. 18 g/mol Na. Cl 58. 44 g/mol Cl 2 70. 90 g/mol (NH 4)3 PO 4 149. 12 g/mol Cu. SO 4· 5 H 2 O 249. 72 g/mol

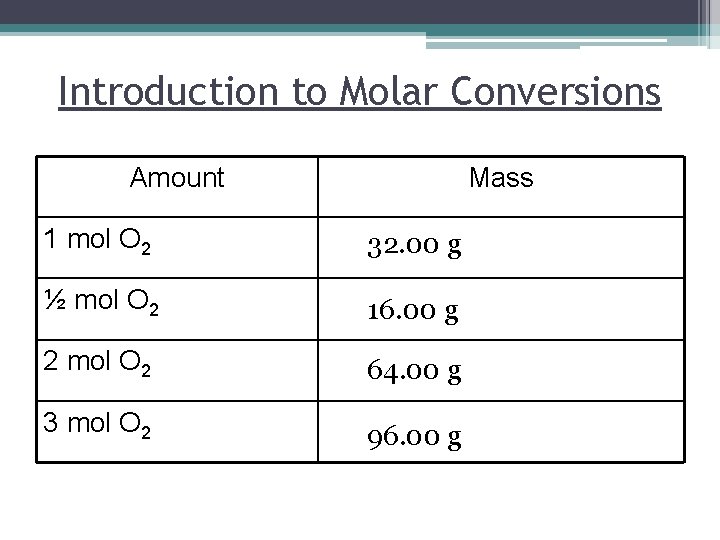
Introduction to Molar Conversions Amount Mass 1 mol O 2 32. 00 g ½ mol O 2 16. 00 g 2 mol O 2 64. 00 g 3 mol O 2 96. 00 g

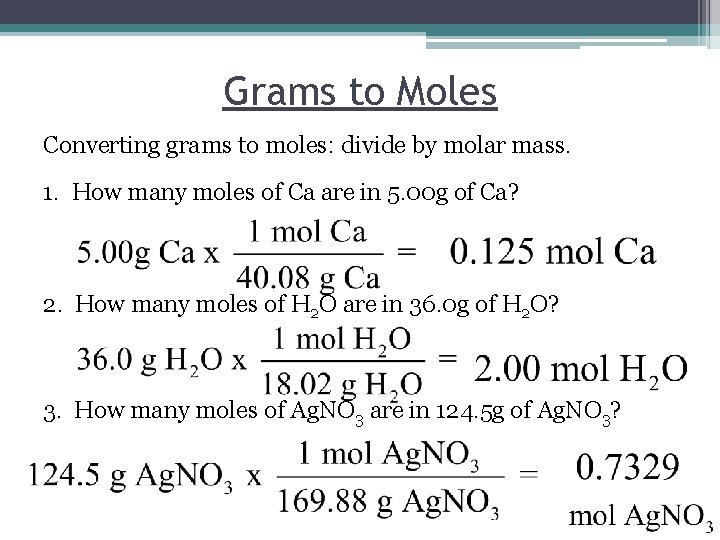
Grams to Moles Converting grams to moles: divide by molar mass. 1. How many moles of Ca are in 5. 00 g of Ca? 2. How many moles of H 2 O are in 36. 0 g of H 2 O? 3. How many moles of Ag. NO 3 are in 124. 5 g of Ag. NO 3?

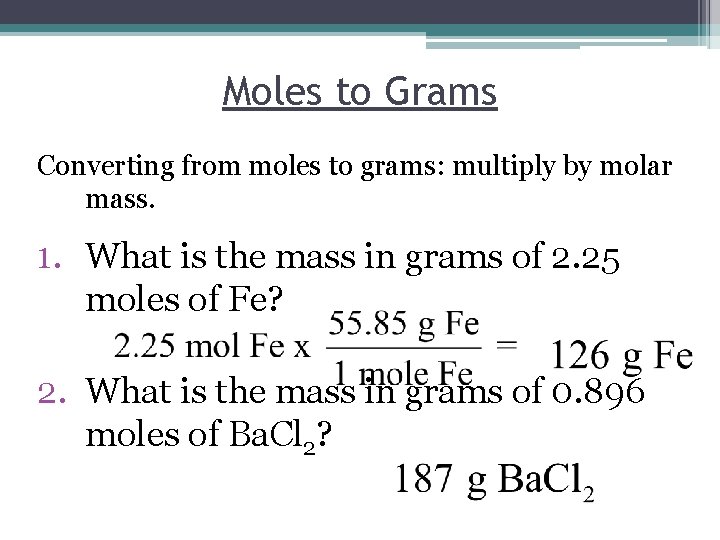
Moles to Grams Converting from moles to grams: multiply by molar mass. 1. What is the mass in grams of 2. 25 moles of Fe? 2. What is the mass in grams of 0. 896 moles of Ba. Cl 2?

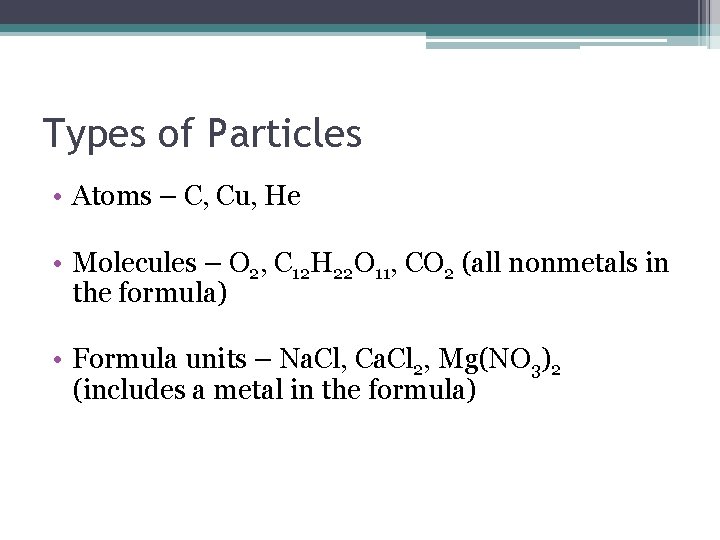
Types of Particles • Atoms – C, Cu, He • Molecules – O 2, C 12 H 22 O 11, CO 2 (all nonmetals in the formula) • Formula units – Na. Cl, Ca. Cl 2, Mg(NO 3)2 (includes a metal in the formula)

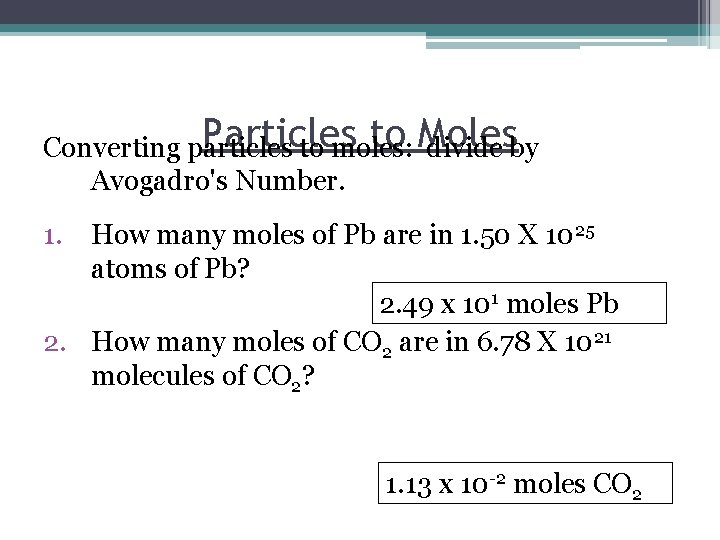
Particles to Moles Converting particles to moles: divide by Avogadro's Number. 1. How many moles of Pb are in 1. 50 X 1025 atoms of Pb? 2. 49 x 101 moles Pb 2. How many moles of CO 2 are in 6. 78 X 1021 molecules of CO 2? 1. 13 x 10 -2 moles CO 2

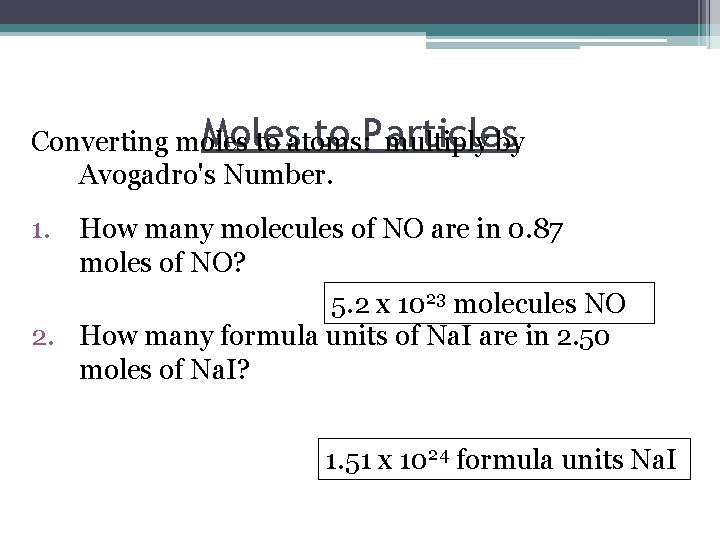
Moles to Particles Converting moles to atoms: multiply by Avogadro's Number. 1. How many molecules of NO are in 0. 87 moles of NO? 5. 2 x 1023 molecules NO 2. How many formula units of Na. I are in 2. 50 moles of Na. I? 1. 51 x 1024 formula units Na. I

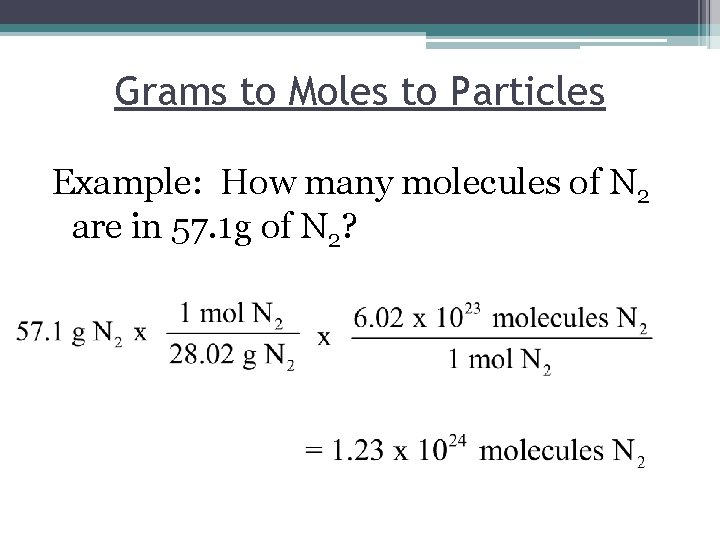
Grams to Moles to Particles Example: How many molecules of N 2 are in 57. 1 g of N 2?

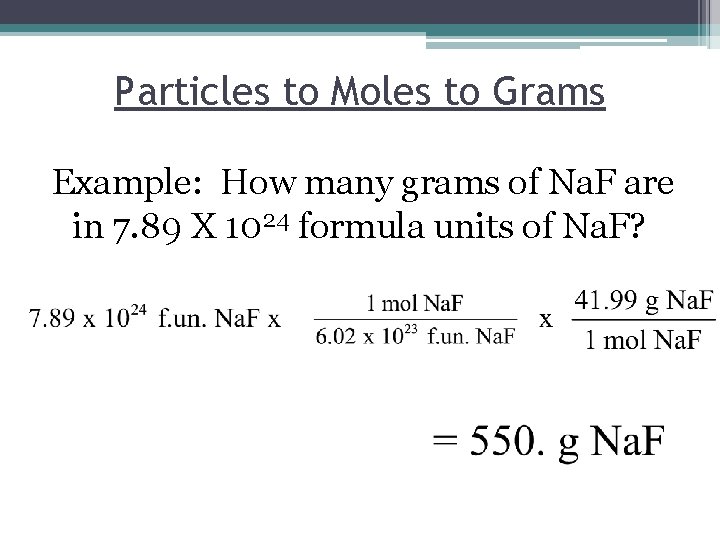
Particles to Moles to Grams Example: How many grams of Na. F are in 7. 89 X 1024 formula units of Na. F?

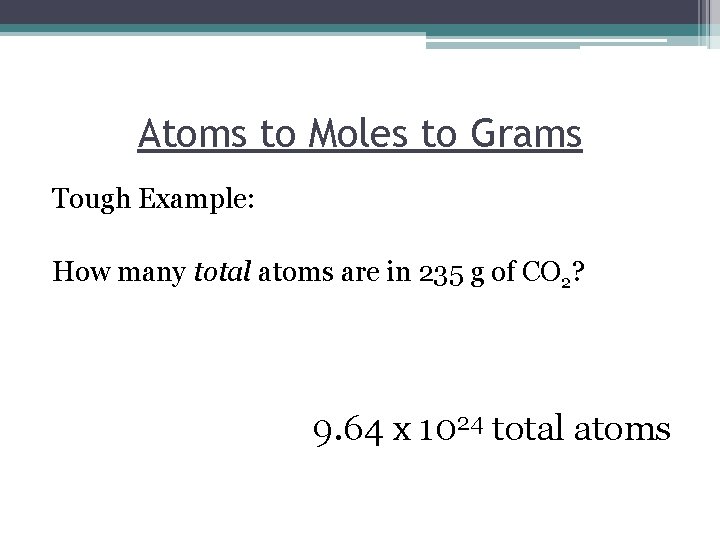
Atoms to Moles to Grams Tough Example: How many total atoms are in 235 g of CO 2? 9. 64 x 1024 total atoms

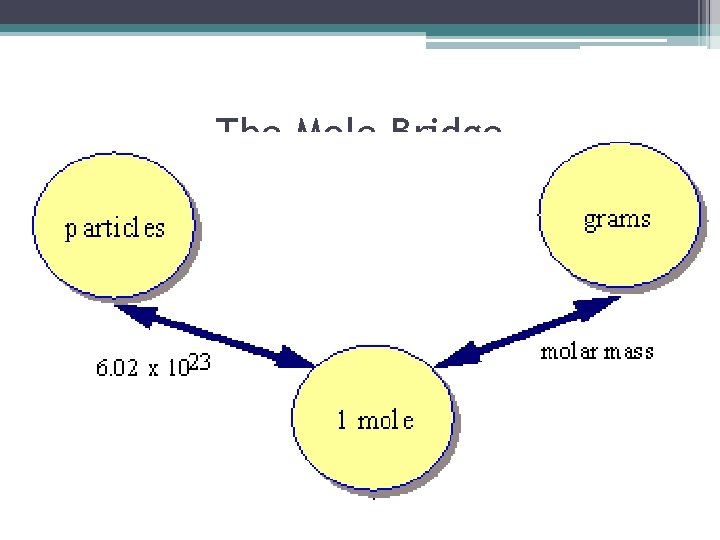
The Mole Bridge