Stoichiometry and the Mole The Scenario You are






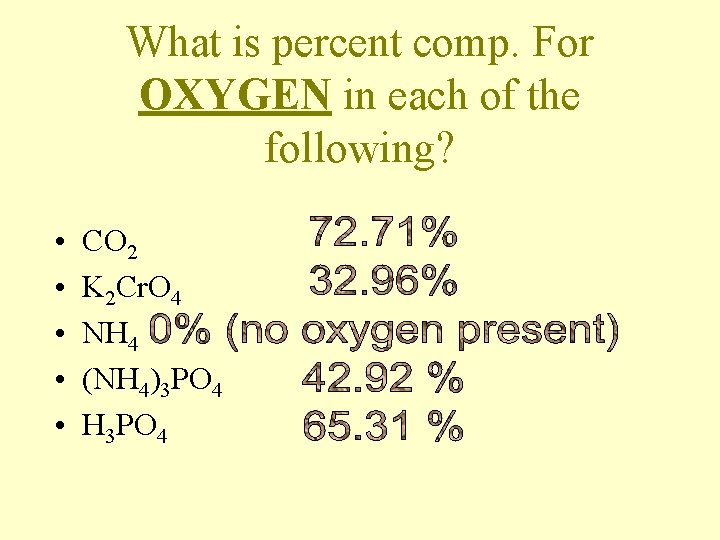




























- Slides: 69

Stoichiometry and the Mole

The Scenario • You are planning to do a reaction using hydrochloric acid. Suddenly, you drop the bottle and spill all 13 grams of your very dangerous acid on the floor! • You know it can be neutralized (so that it stops eating through the floor) by sodium hydroxide, but you need to know how much sodium hydroxide you need to completely get rid of all of the acid. How do you do this? ? ?

The acid spill… • You know that: – HCl + Na. OH ----> Na. Cl + H 2 O – Very dangerous ---> Very harmless • You also know you have 13 g of HCl • Wouldn’t it be nice to know how much one atom of an element weighed! • The big problem is that elements have different isotopes.

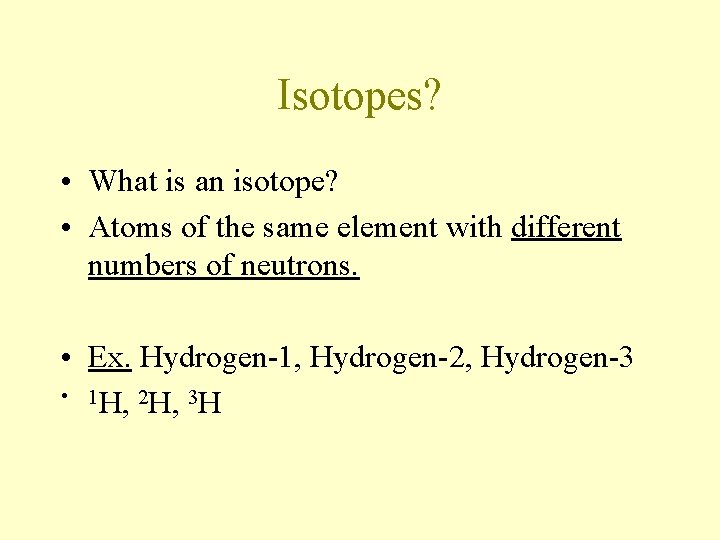
Isotopes? • What is an isotope? • Atoms of the same element with different numbers of neutrons. • Ex. Hydrogen-1, Hydrogen-2, Hydrogen-3 • 1 H, 2 H, 3 H

Average Atomic Mass • The average atomic mass for all elements are shown on the periodic table. • The periodic table shows the AVERAGE weights of all isotopes.

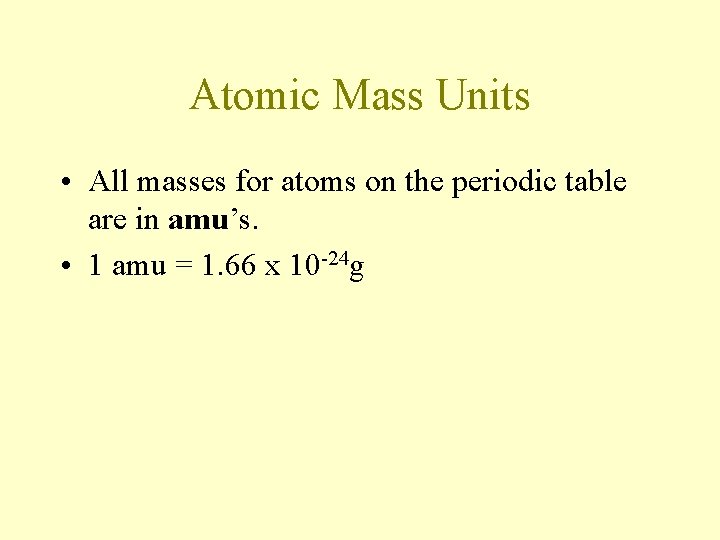
Atomic Mass Units • All masses for atoms on the periodic table are in amu’s. • 1 amu = 1. 66 x 10 -24 g

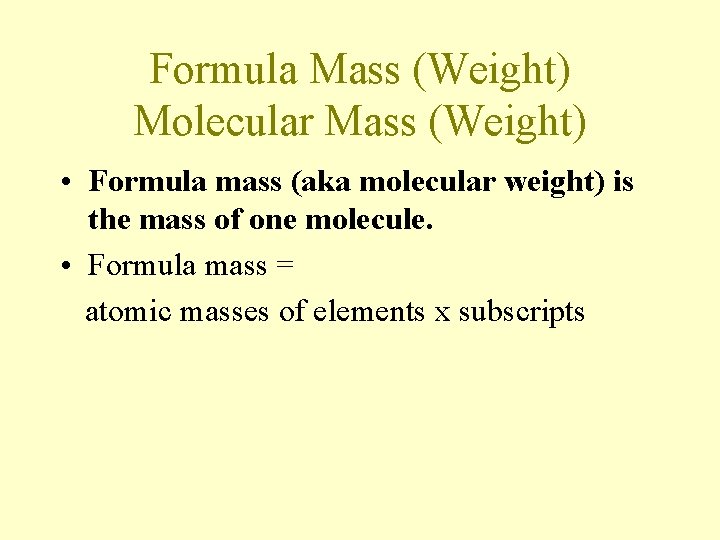
Formula Mass (Weight) Molecular Mass (Weight) • Formula mass (aka molecular weight) is the mass of one molecule. • Formula mass = atomic masses of elements x subscripts

Find the formula mass of water. • • • Water is H 2 O From periodic table H --> 1. 008 amu’s From periodic table O --> 16. 00 amu’s BUT there are 2 H’s and only 1 O in H 2 O Solution: – H --> 2 x 1. 008 = 2. 016 amu + Add these together – O --> 1 x 16. 00 = 16. 00 amu 18. 02 amu

Find the formula masses for the following: • • • CO 2 K 2 Cr. O 4 NH 4 (NH 4)3 PO 4 H 3 PO 4

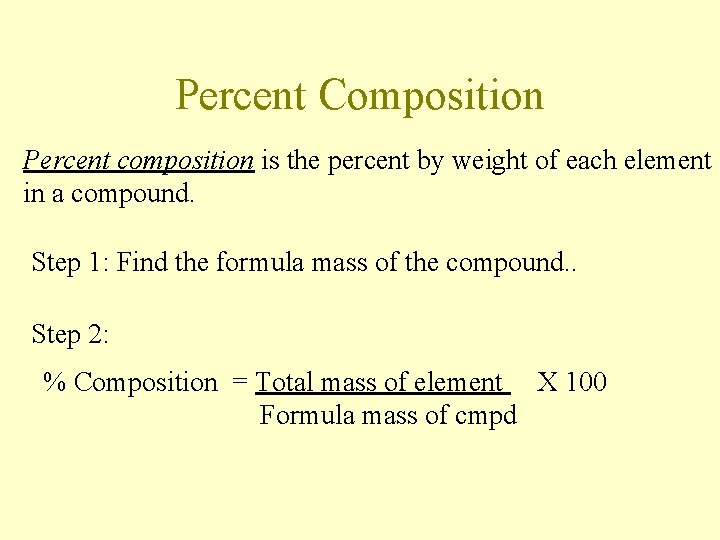
Percent Composition Percent composition is the percent by weight of each element in a compound. Step 1: Find the formula mass of the compound. . Step 2: % Composition = Total mass of element X 100 Formula mass of cmpd

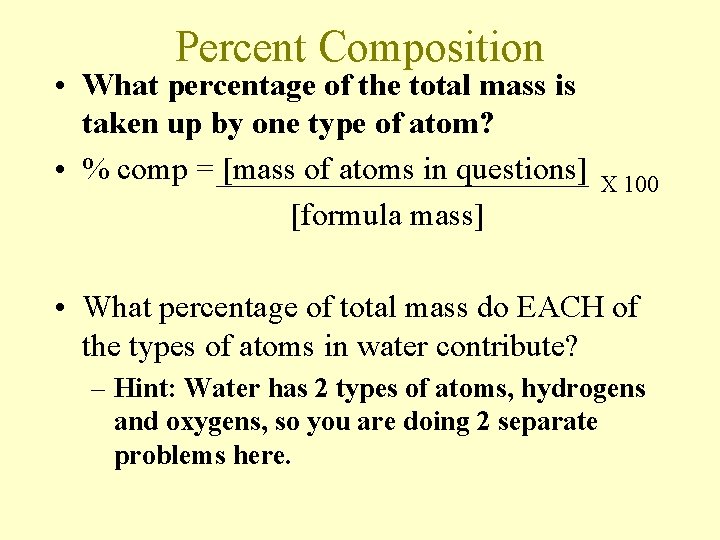
Percent Composition • What percentage of the total mass is taken up by one type of atom? • % comp = [mass of atoms in questions] [formula mass] X 100 • What percentage of total mass do EACH of the types of atoms in water contribute? – Hint: Water has 2 types of atoms, hydrogens and oxygens, so you are doing 2 separate problems here.

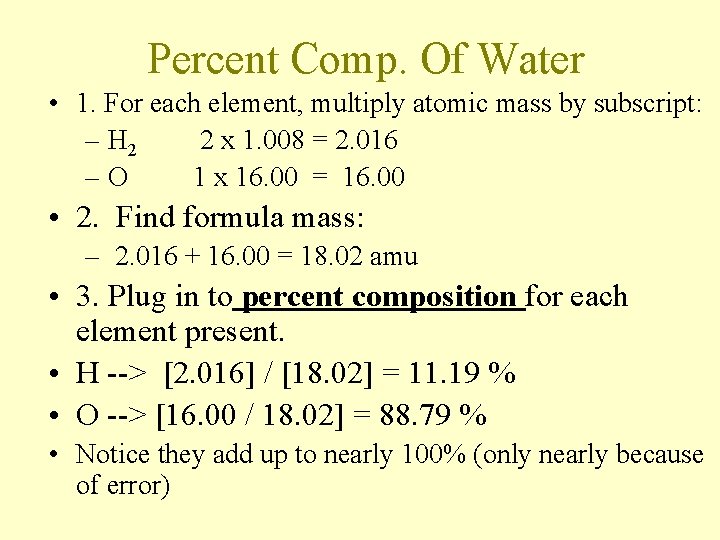
Percent Comp. Of Water • 1. For each element, multiply atomic mass by subscript: – H 2 2 x 1. 008 = 2. 016 –O 1 x 16. 00 = 16. 00 • 2. Find formula mass: – 2. 016 + 16. 00 = 18. 02 amu • 3. Plug in to percent composition for each element present. • H --> [2. 016] / [18. 02] = 11. 19 % • O --> [16. 00 / 18. 02] = 88. 79 % • Notice they add up to nearly 100% (only nearly because of error)
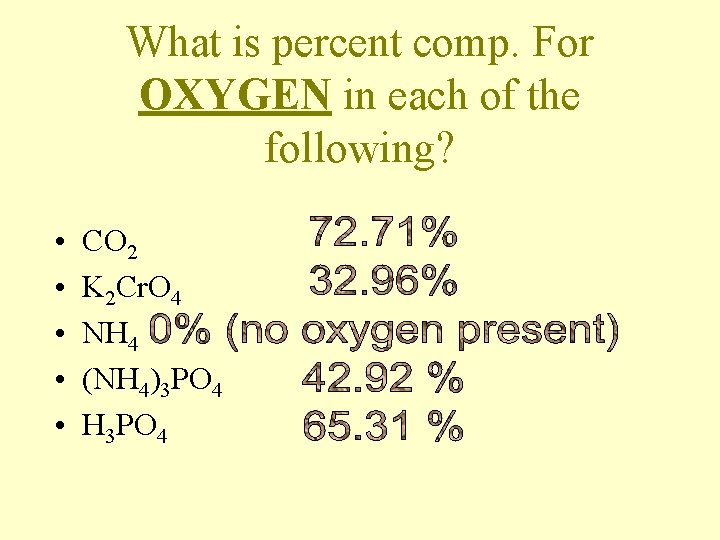
What is percent comp. For OXYGEN in each of the following? • • • CO 2 K 2 Cr. O 4 NH 4 (NH 4)3 PO 4 H 3 PO 4

A Mass Challenge • 1. How many grams of oxygen are in 237 g of H 3 PO 4? • 2. How many grams of hydrogen are in 360 g of (NH 4)3 PO 4? • 3. How many grams of copper are in 296 g of Cu(OH)2?

Introducing…The Mole!!! • 1 mole = approximately 602, 204, 531, 000, 000 • That is… • 602 sextillion • 204 quintillion • 531 quadrillion • plus or minus a few hundred trillion

The Mole • 1 mole = 6. 022 x 1023 particles. • A mole is the “unit if measure. • 6. 02 x 1023 is called Avogadro’s Number.

Avogadro’s Number No! Not avocado - Avogadro!

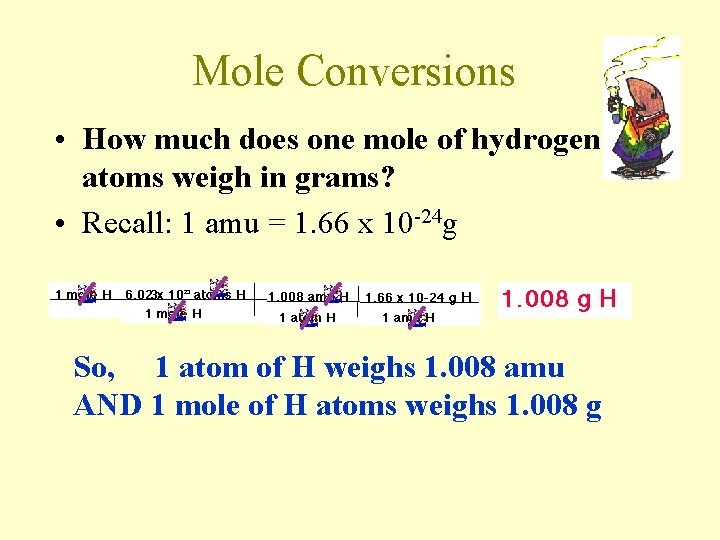
Mole Conversions • How much does one mole of hydrogen atoms weigh in grams? • Recall: 1 amu = 1. 66 x 10 -24 g So, 1 atom of H weighs 1. 008 amu AND 1 mole of H atoms weighs 1. 008 g

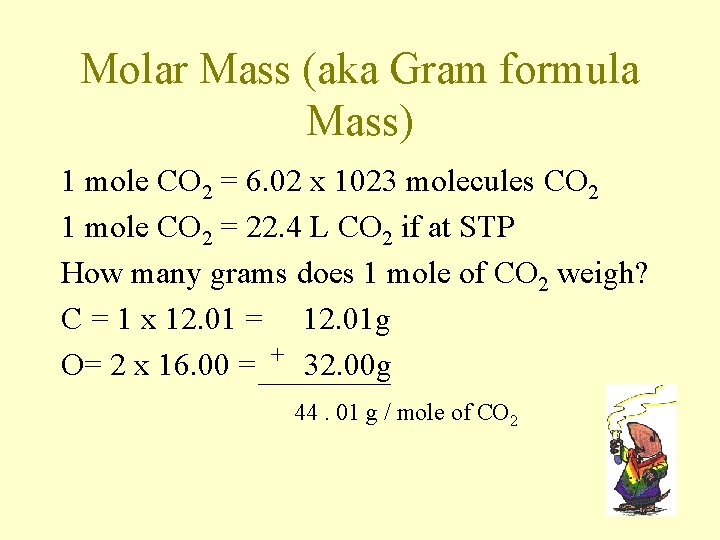
Conversion options with the mole ü 1 mole = 6. 022 x 1023 particles ü 1 mole of atoms = mass on per. table in grams ü 1 mole = 22. 4 L (at STP & for gases only!!) STP is standard Temperature (273 K) & Pressure (1 atm)

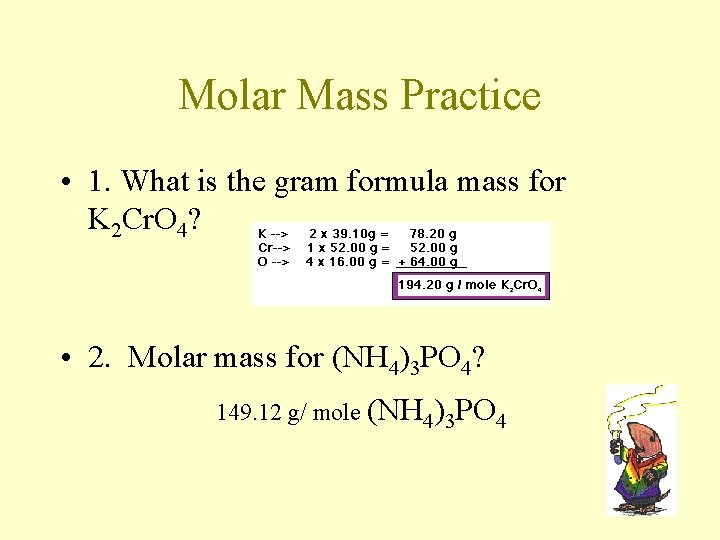
Molar Mass (aka Gram formula Mass) 1 mole CO 2 = 6. 02 x 1023 molecules CO 2 1 mole CO 2 = 22. 4 L CO 2 if at STP How many grams does 1 mole of CO 2 weigh? C = 1 x 12. 01 = 12. 01 g O= 2 x 16. 00 = + 32. 00 g 44. 01 g / mole of CO 2

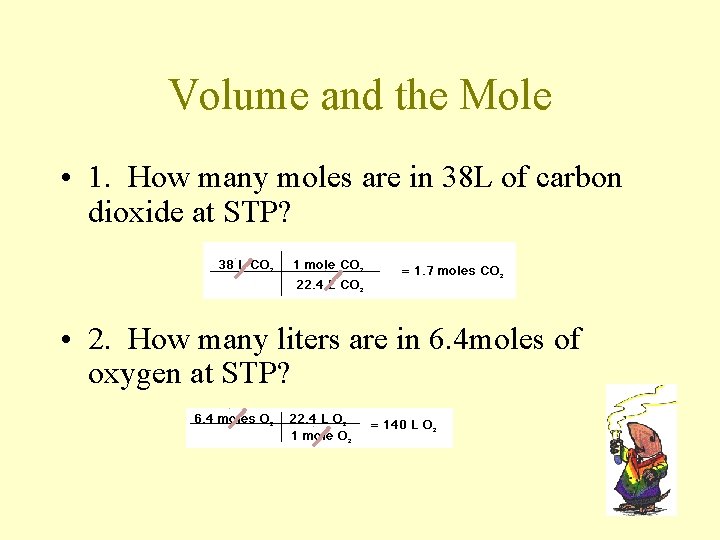
Molar Mass Practice • 1. What is the gram formula mass for K 2 Cr. O 4? • 2. Molar mass for (NH 4)3 PO 4? 149. 12 g/ mole (NH 4)3 PO 4

Volume and the Mole • 1. How many moles are in 38 L of carbon dioxide at STP? • 2. How many liters are in 6. 4 moles of oxygen at STP?

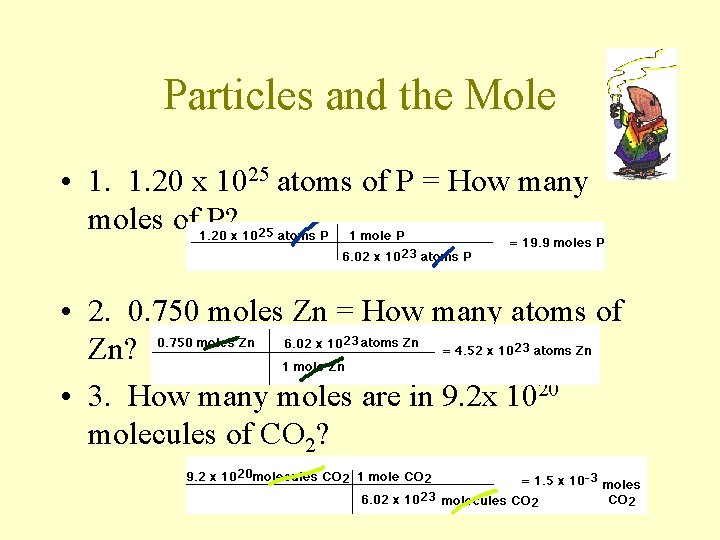
Particles and the Mole • 1. 1. 20 x 1025 atoms of P = How many moles of P? • 2. 0. 750 moles Zn = How many atoms of Zn? • 3. How many moles are in 9. 2 x 1020 molecules of CO 2?


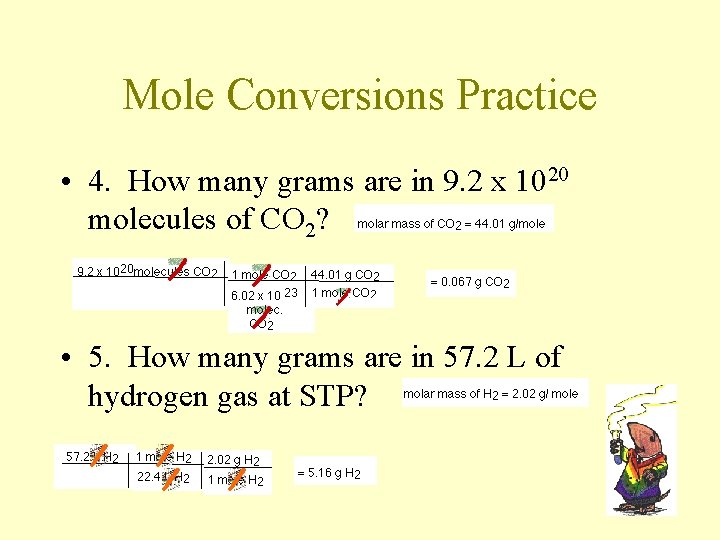
Mole Conversions Practice • 4. How many grams are in 9. 2 x 1020 molecules of CO 2? • 5. How many grams are in 57. 2 L of hydrogen gas at STP?


So what is “Mole. American Airlines” Representing? • Interpreting Chemical Equations • What does a chemical equation ACTUALLY tell you? What does it all mean? • Ex: N 2(g) + 3 H 2(g) ---> 2 NH 3(g) • This says that 1 molecule of N 2 reacts with 3 molecules of H 2 to give 2 molecules of NH 3 gas. • Let’s say you have 1 mole of nitrogen gas and an unlimited supply of hydrogen gas – 1. How much of the hydrogen gas will react with the nitrogen gas? 3 moles – 2. How much ammonia gas can you make? 2 moles

I. Intro to Stoichiometry is the process of calculating the amount of substances produced in a chemical reaction. When you know the amount of one substance, you can determine the amount of the other reactants or products.

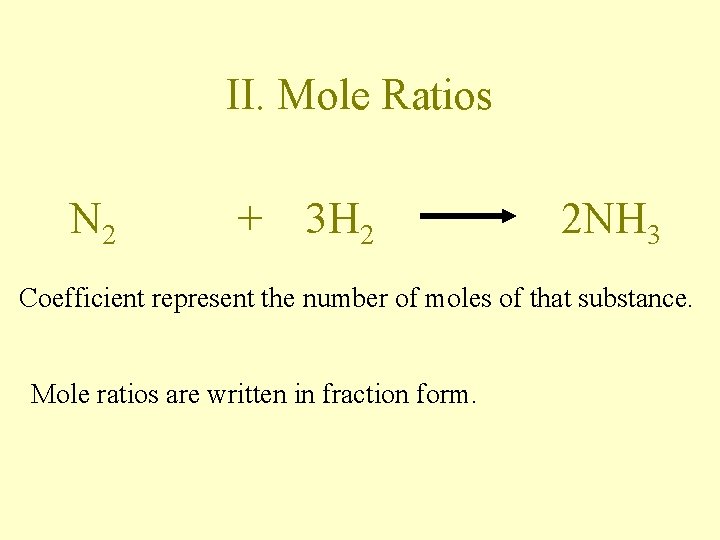
II. Mole Ratios N 2 + 3 H 2 2 NH 3 Coefficient represent the number of moles of that substance. Mole ratios are written in fraction form.

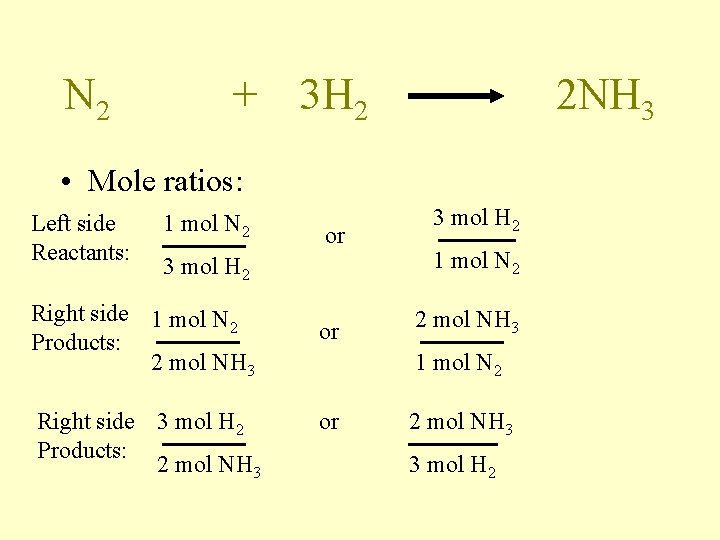
N 2 + 3 H 2 2 NH 3 • Mole ratios: Left side Reactants: 1 mol N 2 or 3 mol H 2 Right side 1 mol N 2 Products: 2 mol NH 3 Right side 3 mol H 2 Products: 2 mol NH 3 or 3 mol H 2 1 mol N 2 2 mol NH 3 1 mol N 2 or 2 mol NH 3 3 mol H 2

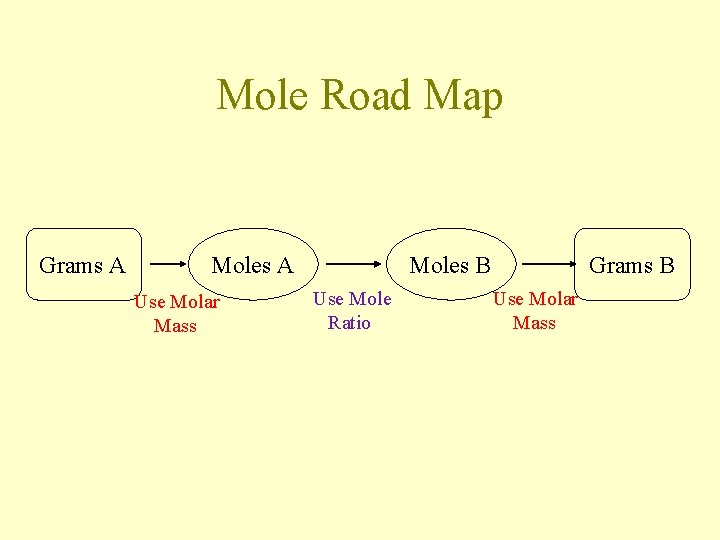
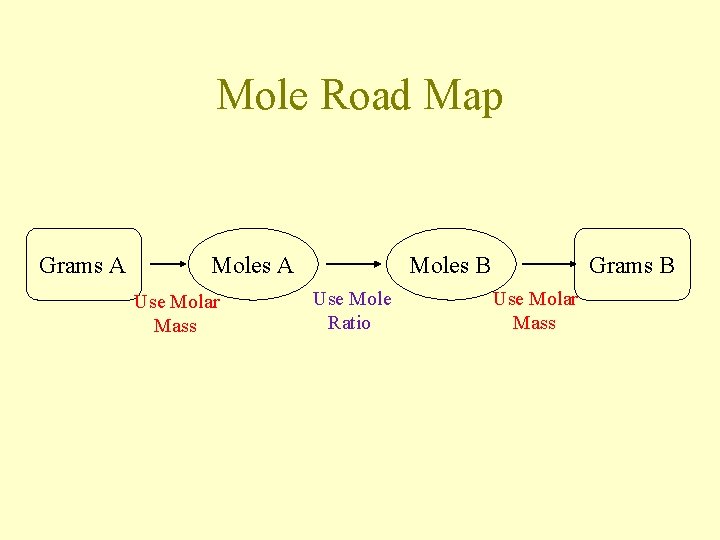
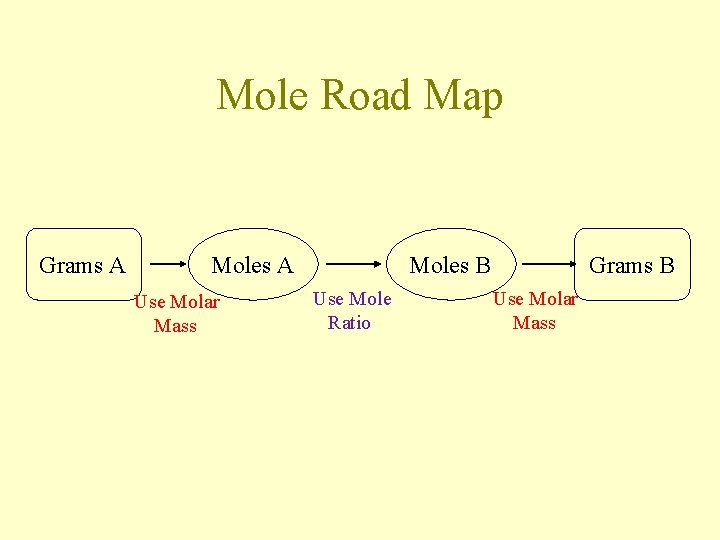
Mole Road Map Grams A Moles A Use Molar Mass Moles B Use Mole Ratio Grams B Use Molar Mass

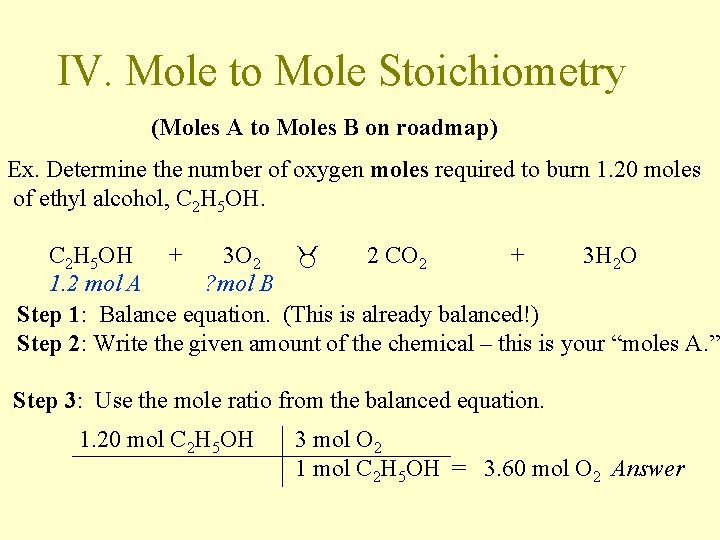
IV. Mole to Mole Stoichiometry (Moles A to Moles B on roadmap) Ex. Determine the number of oxygen moles required to burn 1. 20 moles of ethyl alcohol, C 2 H 5 OH + 3 O 2 2 CO 2 + 3 H 2 O 1. 2 mol A ? mol B Step 1: Balance equation. (This is already balanced!) Step 2: Write the given amount of the chemical – this is your “moles A. ” Step 3: Use the mole ratio from the balanced equation. 1. 20 mol C 2 H 5 OH 3 mol O 2 1 mol C 2 H 5 OH = 3. 60 mol O 2 Answer

Mole Road Map Grams A Moles A Use Molar Mass Moles B Use Mole Ratio Grams B Use Molar Mass

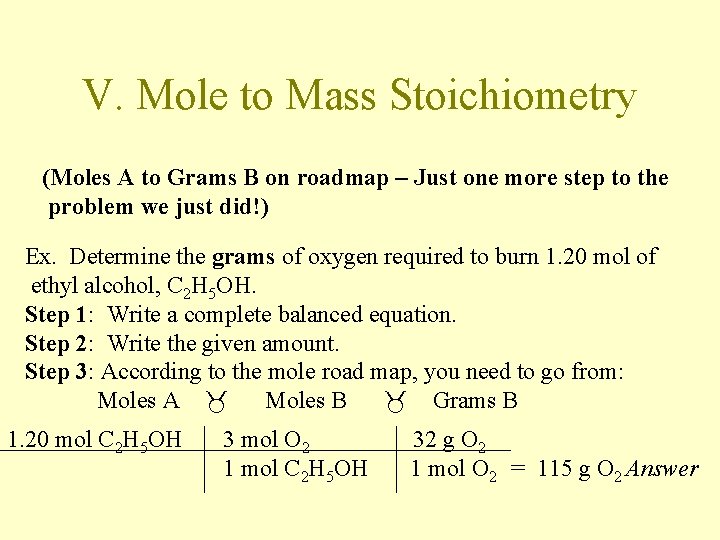
V. Mole to Mass Stoichiometry (Moles A to Grams B on roadmap – Just one more step to the problem we just did!) Ex. Determine the grams of oxygen required to burn 1. 20 mol of ethyl alcohol, C 2 H 5 OH. Step 1: Write a complete balanced equation. Step 2: Write the given amount. Step 3: According to the mole road map, you need to go from: Moles A Moles B Grams B 1. 20 mol C 2 H 5 OH 3 mol O 2 1 mol C 2 H 5 OH 32 g O 2 1 mol O 2 = 115 g O 2 Answer

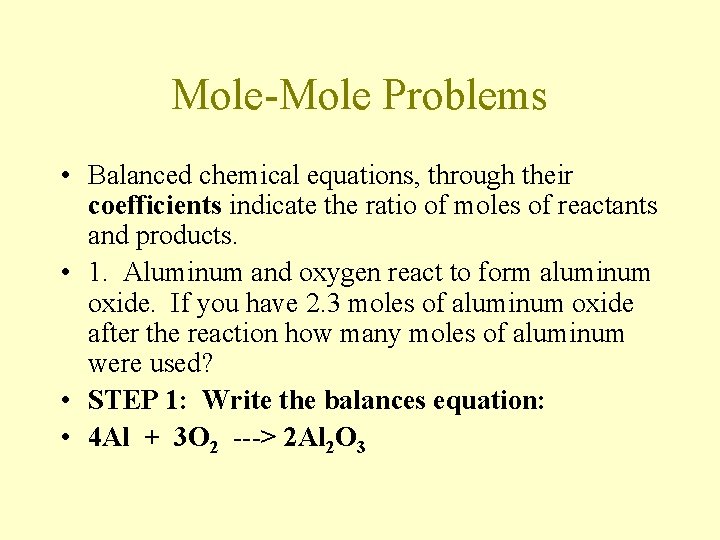
Mole-Mole Problems • Balanced chemical equations, through their coefficients indicate the ratio of moles of reactants and products. • 1. Aluminum and oxygen react to form aluminum oxide. If you have 2. 3 moles of aluminum oxide after the reaction how many moles of aluminum were used? • STEP 1: Write the balances equation: • 4 Al + 3 O 2 ---> 2 Al 2 O 3

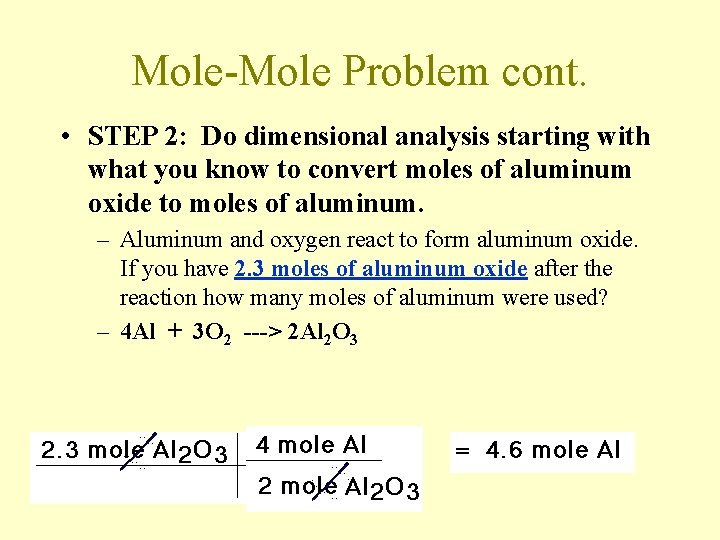
Mole-Mole Problem cont. • STEP 2: Do dimensional analysis starting with what you know to convert moles of aluminum oxide to moles of aluminum. – Aluminum and oxygen react to form aluminum oxide. If you have 2. 3 moles of aluminum oxide after the reaction how many moles of aluminum were used? – 4 Al + 3 O 2 ---> 2 Al 2 O 3

Now you try a mole-mole problem • 2. Hydrogen gas and oxygen gas react to make water. If you have 3. 24 moles of hydrogen gas and an unlimited supply of oxygen gas, how many moles of water can you make? • H 2 + O 2 ---> 2 H 2 O

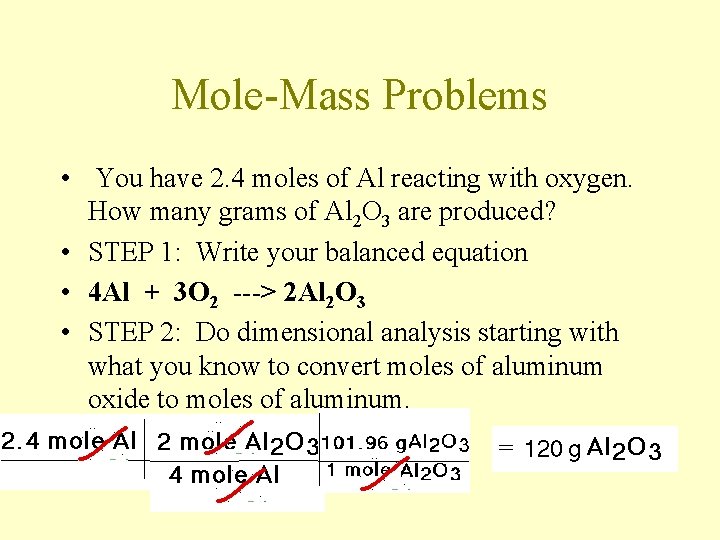
Mole-Mass Problems • You have 2. 4 moles of Al reacting with oxygen. How many grams of Al 2 O 3 are produced? • STEP 1: Write your balanced equation • 4 Al + 3 O 2 ---> 2 Al 2 O 3 • STEP 2: Do dimensional analysis starting with what you know to convert moles of aluminum oxide to moles of aluminum.

Mole-Mass Problems- you try: • You have 6. 7 moles of oxygen gas reacting with unlimited supply of hydrogen. How many grams of water are produced? • H 2 + O 2 ---> 2 H 2 O

Mole Road Map Grams A Moles A Use Molar Mass Moles B Use Mole Ratio Grams B Use Molar Mass

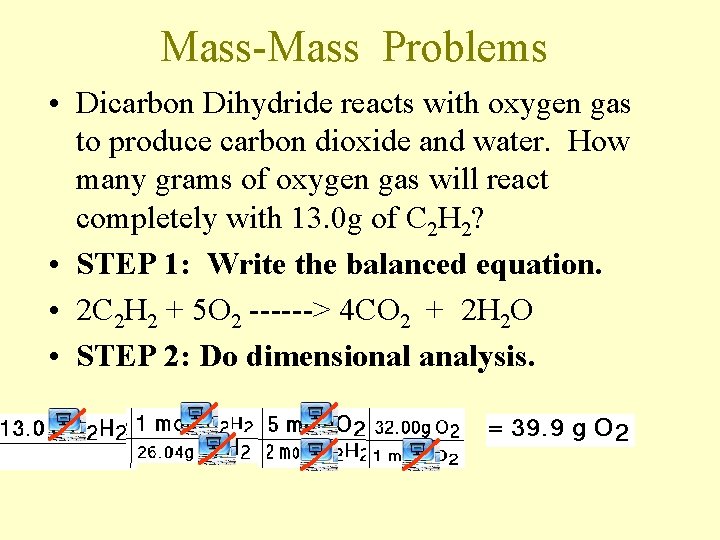
Mass-Mass Problems • Dicarbon Dihydride reacts with oxygen gas to produce carbon dioxide and water. How many grams of oxygen gas will react completely with 13. 0 g of C 2 H 2? • STEP 1: Write the balanced equation. • 2 C 2 H 2 + 5 O 2 ------> 4 CO 2 + 2 H 2 O • STEP 2: Do dimensional analysis.

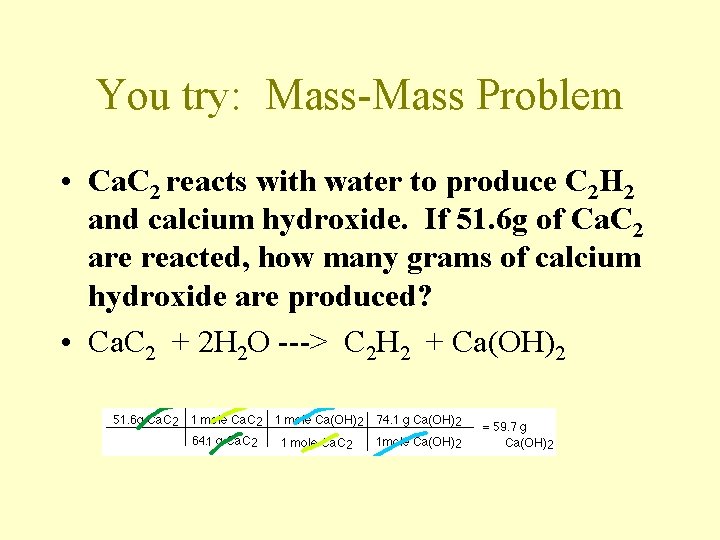
You try: Mass-Mass Problem • Ca. C 2 reacts with water to produce C 2 H 2 and calcium hydroxide. If 51. 6 g of Ca. C 2 are reacted, how many grams of calcium hydroxide are produced? • Ca. C 2 + 2 H 2 O ---> C 2 H 2 + Ca(OH)2

Theoretical, Experimental, & Percent Yields Theoretical yield is the amount of product that you mathematically determine (using stoich) from a chemical reaction. Experimental yield is the amount of product that you actually get when you perform the reaction in lab. Percent yield = Experimental Yield Theoretical Yield x 100

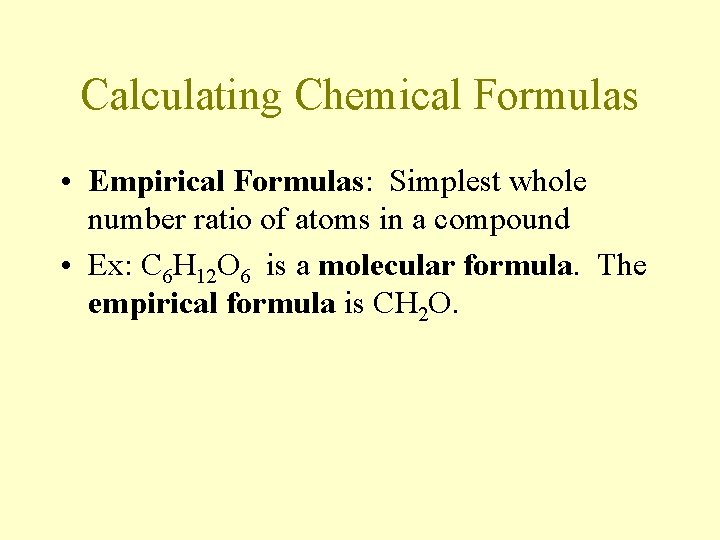
Calculating Chemical Formulas • Empirical Formulas: Simplest whole number ratio of atoms in a compound • Ex: C 6 H 12 O 6 is a molecular formula. The empirical formula is CH 2 O.

Determining Empirical Formulas • Step One: Convert grams to moles. • Step Two: Divide each by the smallest number of moles to get subscripts • Step Three: If you do not have a whole number, or one that is reasonably close, multiply by an integer to obtain a whole number.

Empirical Formula • Hg--> 67. 6 g x 1 mole =. 337 moles Hg /. 337 = 1 200. 59 g • S --> 10. 8 g x 1 mole =. 337 moles S /. 337 = 1 32. 06 g • O --> 21. 6 g x 1 mole = 1. 35 moles O /. 337 = 4 16. 00 g Now divide each by the smallest number of moles Hg. SO 4 These are your subscripts for your formula!

Empirical Formula from % cont… • Problem 2: After doing several experiments on an unknown substance it was found that the percent compositions for the compound were: 3. 09% H, 31. 6% P, and 65. 3 % O. What is the empirical formula for this substance? H 3 PO 4

Empirical Formulas if given mass. • STEP 1: Turn mass into moles • STEP 2: Divide by lowest number of moles to get subscripts. • Problem 1: A 19. 12 gram sample of an unknown chemical was discovered. After much experimentation it was determined that the unknown was made of 3. 03 g of hydrogen and 16. 09 g of oxygen. What is the empirical formula? H 3 O

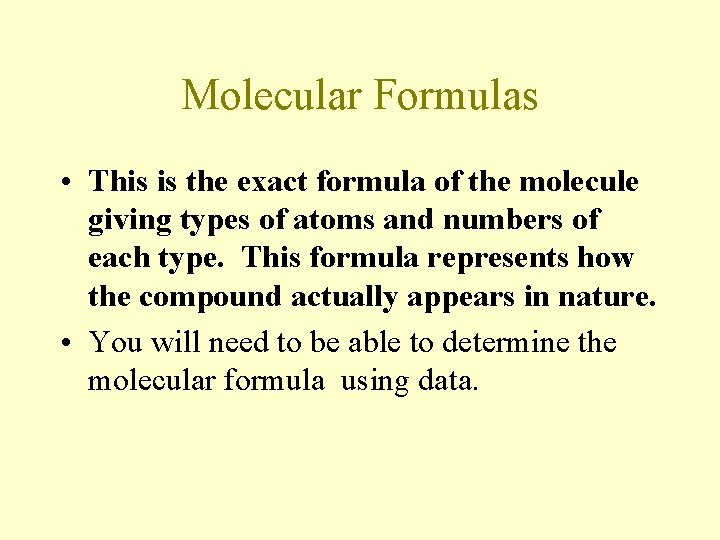
Molecular Formulas • This is the exact formula of the molecule giving types of atoms and numbers of each type. This formula represents how the compound actually appears in nature. • You will need to be able to determine the molecular formula using data.

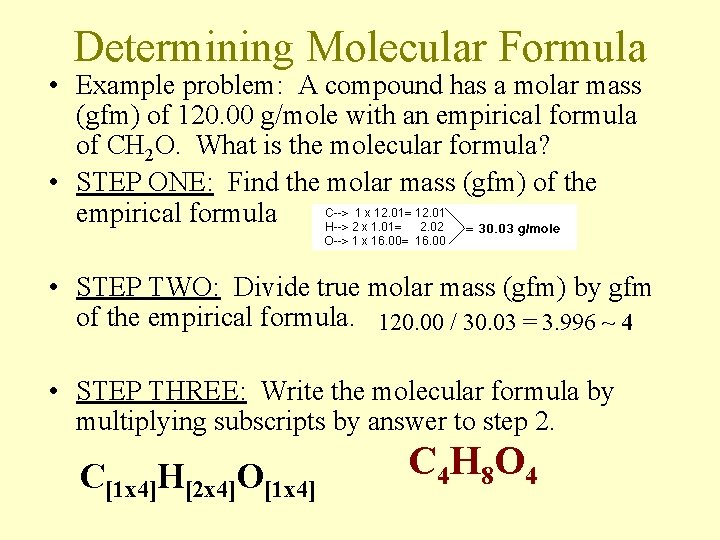
Determining Molecular Formula • Example problem: A compound has a molar mass (gfm) of 120. 00 g/mole with an empirical formula of CH 2 O. What is the molecular formula? • STEP ONE: Find the molar mass (gfm) of the empirical formula • STEP TWO: Divide true molar mass (gfm) by gfm of the empirical formula. 120. 00 / 30. 03 = 3. 996 ~ 4 • STEP THREE: Write the molecular formula by multiplying subscripts by answer to step 2. C[1 x 4]H[2 x 4]O[1 x 4] C 4 H 8 O 4

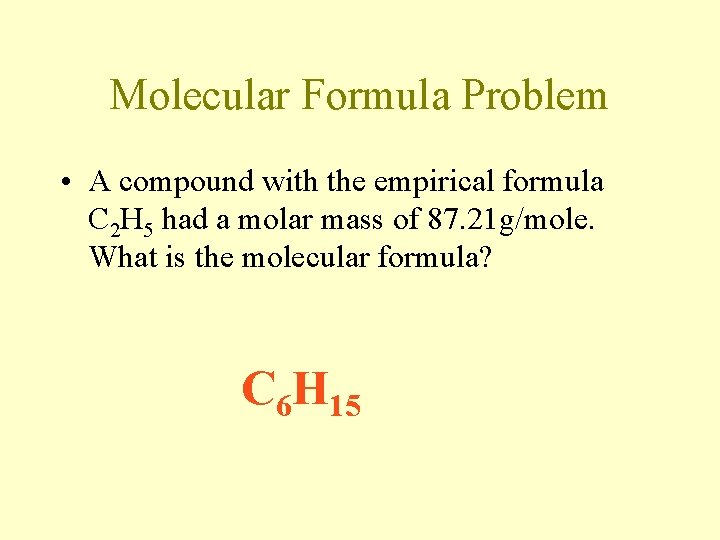
Molecular Formula Problem • A compound with the empirical formula C 2 H 5 had a molar mass of 87. 21 g/mole. What is the molecular formula? C 6 H 15

Hydrated Compounds • A Hydrated Compound is a compound that crystallized from a water solution with water molecules adhering to the particles of the crystal. • Chemists use heat to dry these compounds and then calculate the ratio of the compound to water.

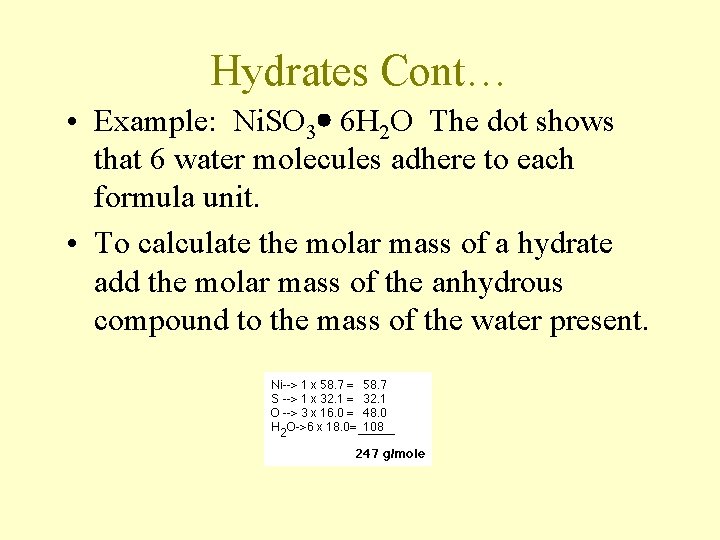
Hydrates Cont… • Example: Ni. SO 3 6 H 2 O The dot shows that 6 water molecules adhere to each formula unit. • To calculate the molar mass of a hydrate add the molar mass of the anhydrous compound to the mass of the water present.

Empirical Formulas of Hydrates • Step 1: Find the moles of anhydride (compound without water) • Step 2: Find the moles of water. • Step 3: Divide moles of water by moles of anhydride. This should turn out to be a whole number • Step 4: Place this whole number into the formula in front of water.

Empirical formula of a hydrate practice • What is the formula of a hydrate which consists of 0. 391 g of Li 2 Si. F 6 and 0. 0903 g of water? • Your formula will be Li 2 Si. F 6 ____H 2 O • Step 1: . 391 g Li 2 Si. F 6 x 1 mole Li 2 Si. F 6 =. 00250 mole 155. 97 g Li 2 Si. F 6 • Step 2: . 0903 g H 2 O x 1 mole H 2 O =. 00500 mole H 2 O 18. 02 g H 2 O • Step 3: . 005 mole /. 0025 mole ~ 2 Li 2 Si. F 6 2 H 2 O

% water in a Hydrate • Step 1: Find the molar mass of the hydrated compound. • Step 2: Divide the mass of water by the mass of the hydrate and multiply by 100.

Hydrates: Practice Problem • What is the formula and % water of a hydrate which consists of 9. 520 g of Ba. I 2 and 0. 887 g of water? Ba. I 2 2 H 2 O 8. 43%

It’s the Holidays… Lets talk Cake! • Let’s say you want to make as many cakes as you can for the holiday season. You don’t have time to go to the store, so you take inventory of what you have in your pantry.

Cake Inventory • • You Have: 1 dozen eggs 5 cups oil 12 cups flour 3 cups sugar 4 boxes cake mix Unlimited supply of water • • Each cake needs: 1 egg 1/3 cup oil 1 cup flour 1/2 cup sugar 1 box cake mix 1/2 cup water How many cakes can we make? 4 What ingredient limits us? Cake mix





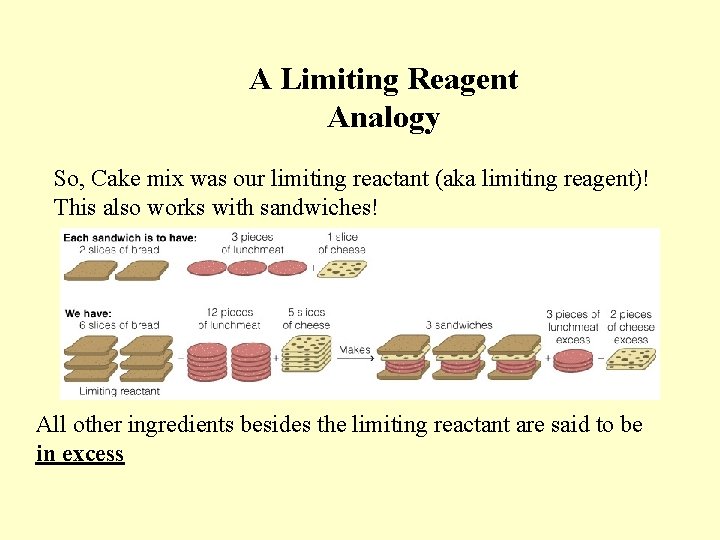
A Limiting Reagent Analogy So, Cake mix was our limiting reactant (aka limiting reagent)! This also works with sandwiches! All other ingredients besides the limiting reactant are said to be in excess

Limiting Reactants Limiting reactant is the chemical in a reaction that restricts how much you can make, it’s the reactant that runs out first. STEP 1: Write the balanced equation! STEP 2: Find desired product in grams. You Will need to do 2 stoich problems. STEP 3: The reactant that gives you the smaller amount of product is the limiting reactant. Hint: You will know if you are working with a limiting reactant problem if you are given two quantities of two reactants or if one is given in excess.

Working Limiting Reactant Problems • Ex: How many grams of lithium nitride can be made by reacting 56. 0 g of nitrogen with 56. 0 g of lithium? • STEP 1: Write the balanced equation! • N 2 + 6 Li ----> 2 Li 3 N • STEP 2: Convert each reactant to what you are looking for.

Final steps of LR problems • STEP 3: The smallest amount found is the maximum amount you can produce using the materials you have. • The limiting reactant is the reactant that gives you the least amount of product • So, Li is my limiting reactant and I can make a maximum of 93. 7 g Li 3 N.

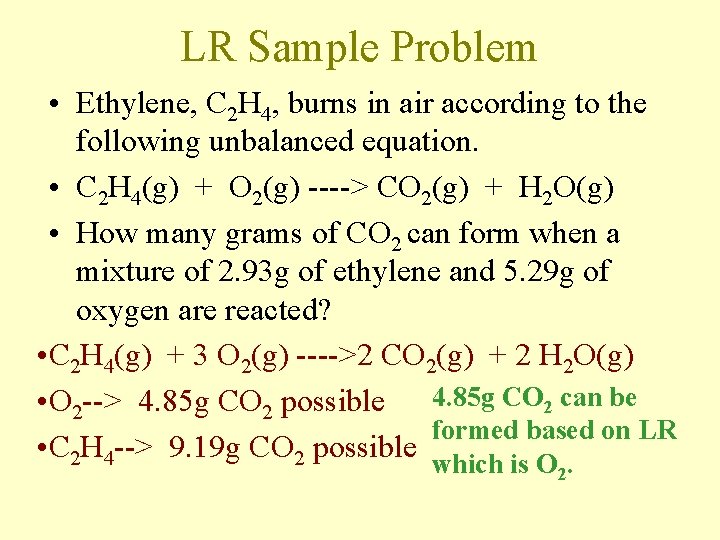
LR Sample Problem • Ethylene, C 2 H 4, burns in air according to the following unbalanced equation. • C 2 H 4(g) + O 2(g) ----> CO 2(g) + H 2 O(g) • How many grams of CO 2 can form when a mixture of 2. 93 g of ethylene and 5. 29 g of oxygen are reacted? • C 2 H 4(g) + 3 O 2(g) ---->2 CO 2(g) + 2 H 2 O(g) • O 2 --> 4. 85 g CO 2 possible 4. 85 g CO 2 can be formed based on LR • C 2 H 4 --> 9. 19 g CO 2 possible which is O 2.

LR Sample #2 • If 40. 0 g of a Pb(NO 3)2 solution are mixed with 37. 0 g of a Na. I solution, how many grams of bright yellow Pb. I 2 form? ØPb(NO 3)2 + 2 Na. I ---> Pb. I 2 + 2 Na. NO 3 Ø Pb(NO 3)2 ---> 55. 7 g Pb. I 2 possible ØNa. I ---> 56. 9 g Pb. I 2 possible ØSo, Pb(NO 3)2 is our L. R. ØWe make 55. 7 g of Pb. I 2