Mole Ratios Calculations with Equations 1 MoleMole Factor





- Slides: 19

Mole Ratios Calculations with Equations 1

Mole-Mole Factor l Shows the mole-to-mole ratio between two of the substances in a balanced equation l Derived from the coefficients of any two substances in the equation 2

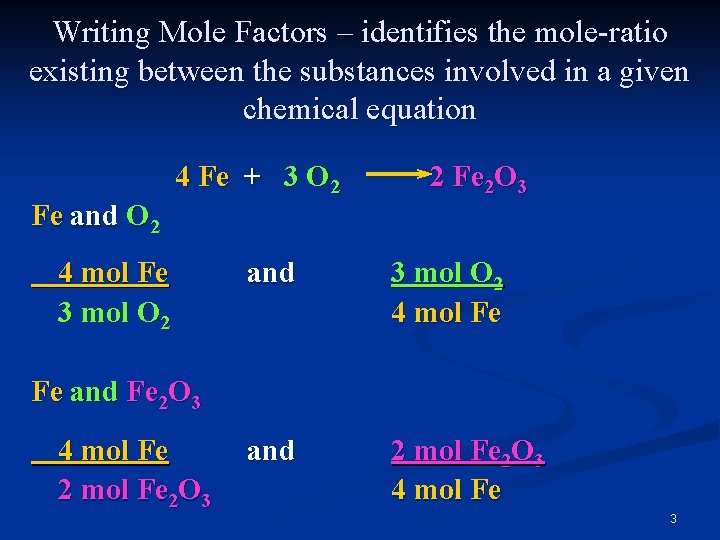
Writing Mole Factors – identifies the mole-ratio existing between the substances involved in a given chemical equation 4 Fe + 3 O 2 2 Fe 2 O 3 Fe and O 2 4 mol Fe 3 mol O 2 and 3 mol O 2 4 mol Fe and 2 mol Fe 2 O 3 4 mol Fe Fe and Fe 2 O 3 4 mol Fe 2 O 3 3

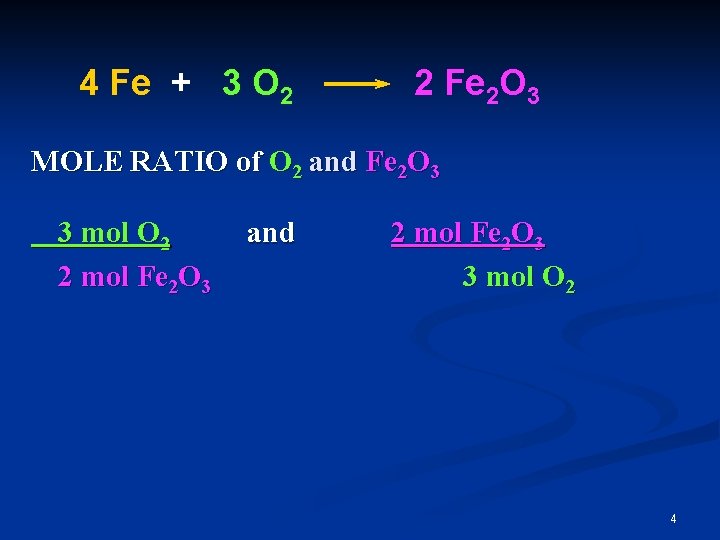
4 Fe + 3 O 2 2 Fe 2 O 3 MOLE RATIO of O 2 and Fe 2 O 3 3 mol O 2 2 mol Fe 2 O 3 and 2 mol Fe 2 O 3 3 mol O 2 4

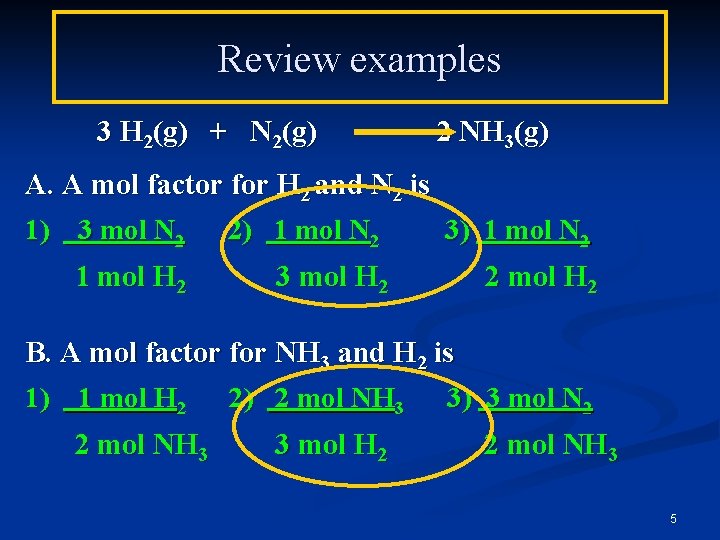
Review examples 3 H 2(g) + N 2(g) 2 NH 3(g) A. A mol factor for H 2 and N 2 is 1) 3 mol N 2 2) 1 mol N 2 3) 1 mol N 2 1 mol H 2 3 mol H 2 2 mol H 2 B. A mol factor for NH 3 and H 2 is 1) 1 mol H 2 2) 2 mol NH 3 3) 3 mol N 2 2 mol NH 3 3 mol H 2 2 mol NH 3 5

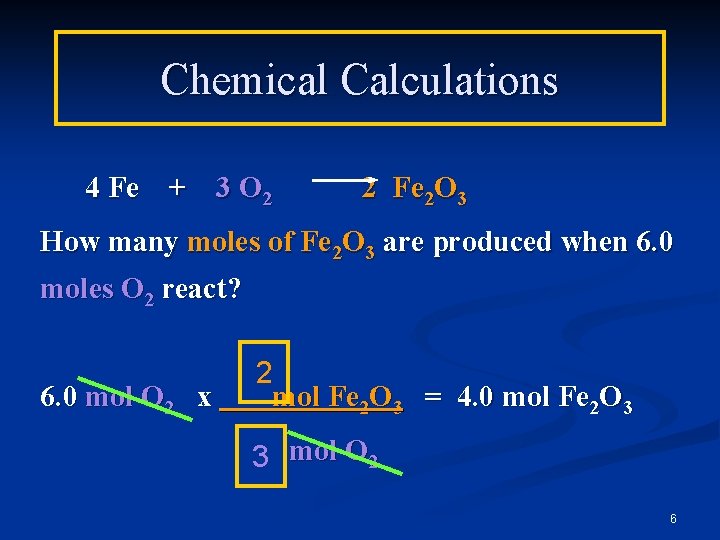
Chemical Calculations 4 Fe + 3 O 2 2 Fe 2 O 3 How many moles of Fe 2 O 3 are produced when 6. 0 moles O 2 react? 6. 0 mol O 2 x 2 mol Fe 2 O 3 = 4. 0 mol Fe 2 O 3 3 mol O 2 6

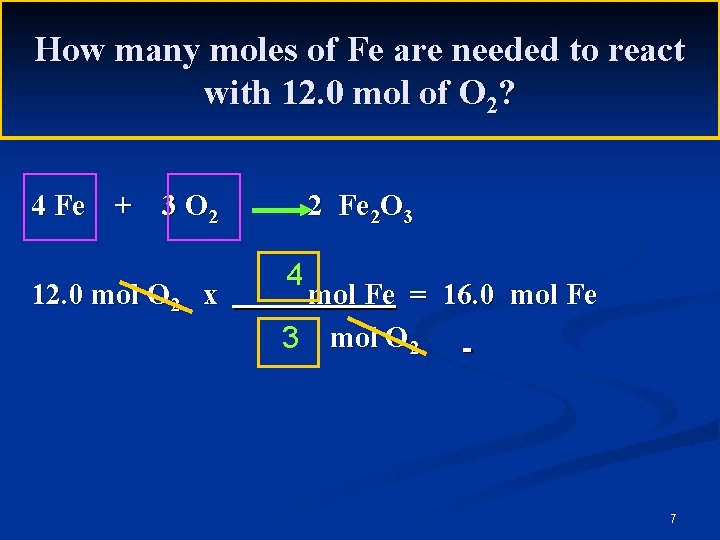
How many moles of Fe are needed to react with 12. 0 mol of O 2? 4 Fe + 3 O 2 12. 0 mol O 2 x 2 Fe 2 O 3 4 mol Fe = 16. 0 mol Fe 3 mol O 2 7

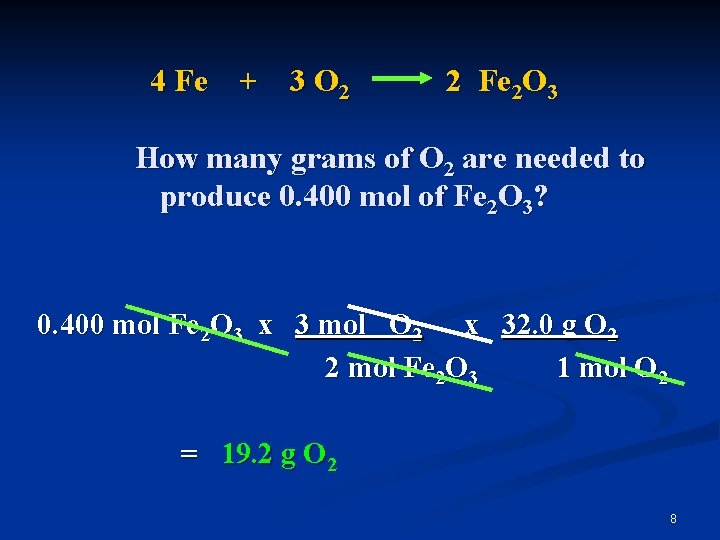
4 Fe + 3 O 2 2 Fe 2 O 3 How many grams of O 2 are needed to produce 0. 400 mol of Fe 2 O 3? 0. 400 mol Fe 2 O 3 x 3 mol O 2 x 32. 0 g O 2 2 mol Fe 2 O 3 1 mol O 2 = 19. 2 g O 2 8

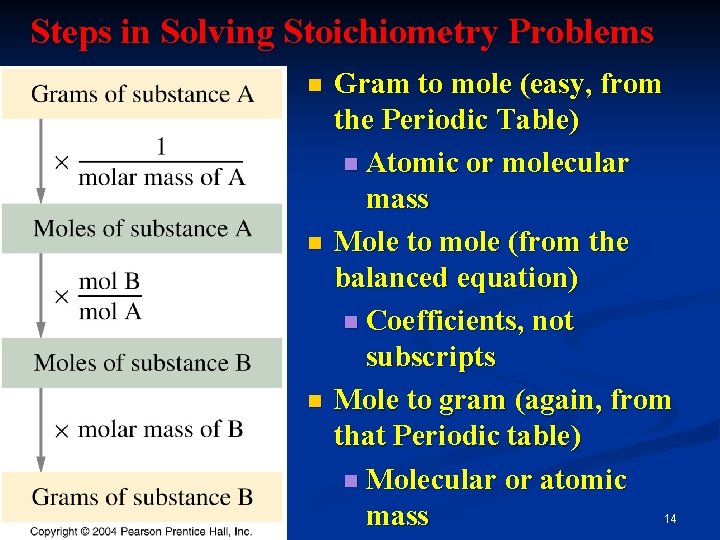
Calculating Mass of A Substance in a Stoichiometric Equation n Balance equation n Convert starting amount to moles n Use coefficients to write a mol-mol factor n Convert moles of desired to grams 9

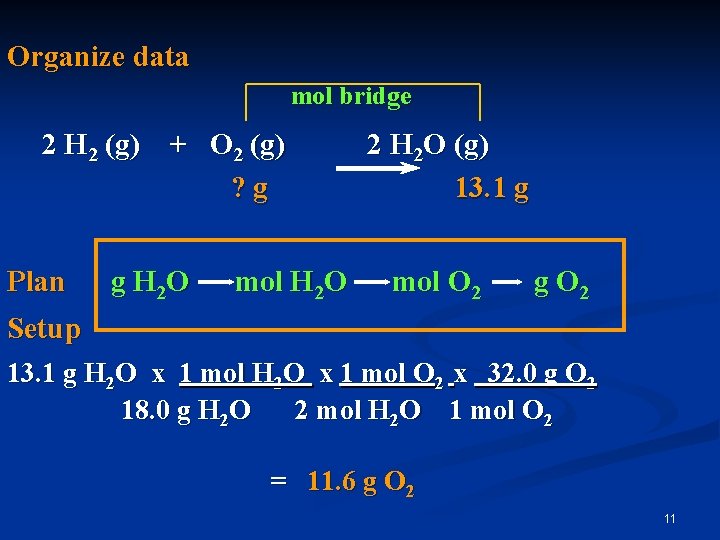
Calculation The reaction between H 2 and O 2 produces 13. 1 g of water. How many grams of O 2 reacted? Write the equation H 2 (g) + O 2 (g) H 2 O (g) Balance the equation 2 H 2 (g) + O 2 (g) 2 H 2 O (g) 10

Organize data mol bridge 2 H 2 (g) + O 2 (g) ? g Plan g H 2 O mol H 2 O 2 H 2 O (g) 13. 1 g mol O 2 g O 2 Setup 13. 1 g H 2 O x 1 mol O 2 x 32. 0 g O 2 18. 0 g H 2 O 2 mol H 2 O 1 mol O 2 = 11. 6 g O 2 11

How many O 2 molecules will react with 505 grams of Na to form Na 2 O? 4 Na + O 2 2 Na 2 O 505 g Na x 1 mol O 2 x 6. 02 x 1023 23. 0 g Na 4 mol Na 1 mol O 2 = 3. 30 x 1024 molecules 12

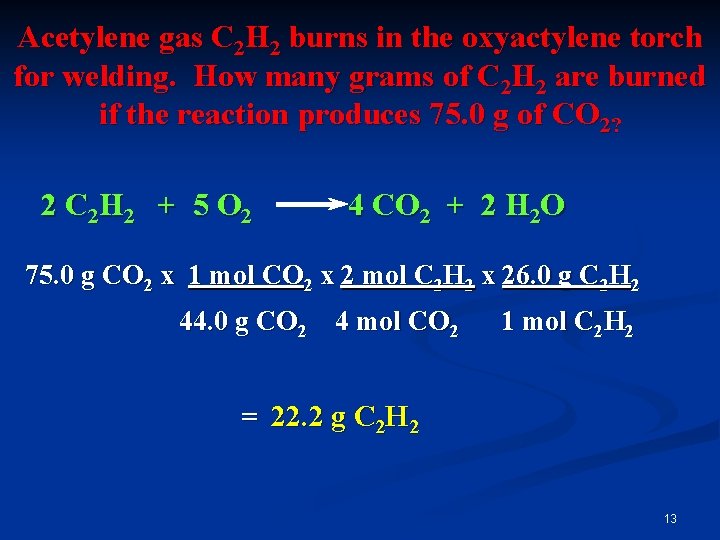
Acetylene gas C 2 H 2 burns in the oxyactylene torch for welding. How many grams of C 2 H 2 are burned if the reaction produces 75. 0 g of CO 2? 2 C 2 H 2 + 5 O 2 4 CO 2 + 2 H 2 O 75. 0 g CO 2 x 1 mol CO 2 x 2 mol C 2 H 2 x 26. 0 g C 2 H 2 44. 0 g CO 2 4 mol CO 2 1 mol C 2 H 2 = 22. 2 g C 2 H 2 13

Steps in Solving Stoichiometry Problems n n n Gram to mole (easy, from the Periodic Table) n Atomic or molecular mass Mole to mole (from the balanced equation) n Coefficients, not subscripts Mole to gram (again, from that Periodic table) n Molecular or atomic mass 14

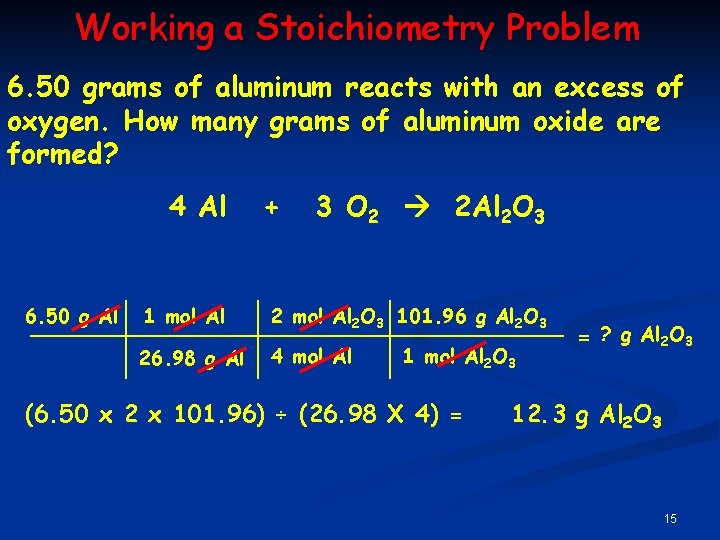
Working a Stoichiometry Problem 6. 50 grams of aluminum reacts with an excess of oxygen. How many grams of aluminum oxide are formed? 4 Al 6. 50 g Al + 3 O 2 2 Al 2 O 3 1 mol Al 2 O 3 101. 96 g Al 2 O 3 26. 98 g Al 4 mol Al 1 mol Al 2 O 3 (6. 50 x 2 x 101. 96) ÷ (26. 98 X 4) = = ? g Al 2 O 3 12. 3 g Al 2 O 3 15

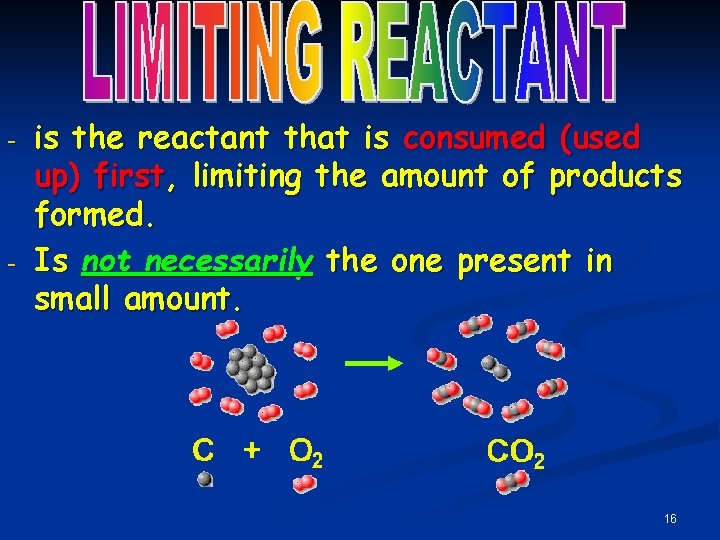
- - is the reactant that is consumed (used up) first, limiting the amount of products formed. Is not necessarily the one present in small amount. 16

HAM SANDWICH EXAMPLE One sandwich is made from one slice of Ham, one slice of Cheese and two slices of Bread 1 H + 1 C + 2 B 1 sandwich How many ham sandwiches can be made from six slices of ham, seven slices of cheese and 14 slices of bread? limiting reactant! 17

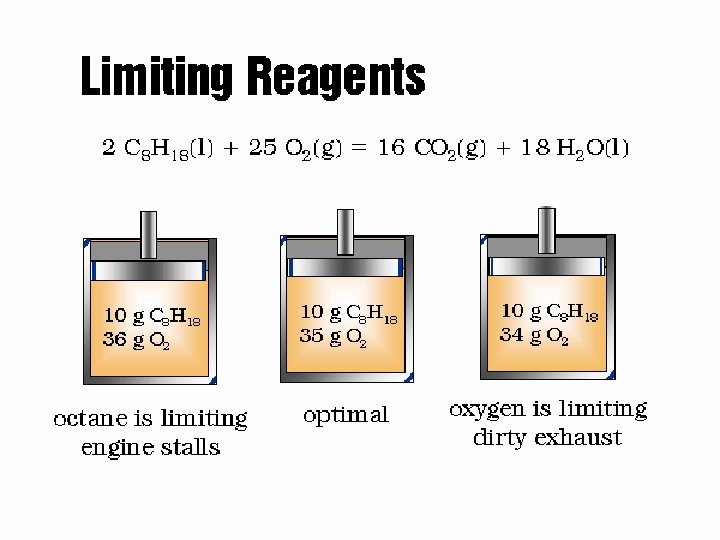
Limiting Reagents - Combustion 18

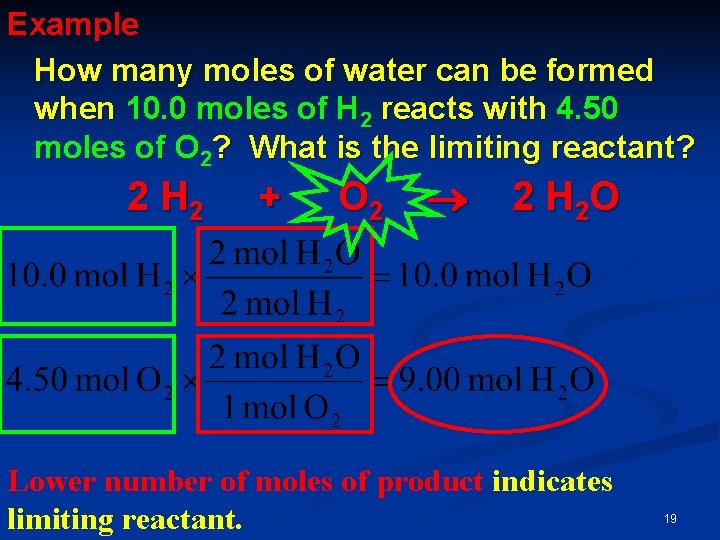
Example How many moles of water can be formed when 10. 0 moles of H 2 reacts with 4. 50 moles of O 2? What is the limiting reactant? 2 H 2 + O 2 2 H 2 O Lower number of moles of product indicates limiting reactant. 19