Mole relationships in chemical equations DONOW Silent and










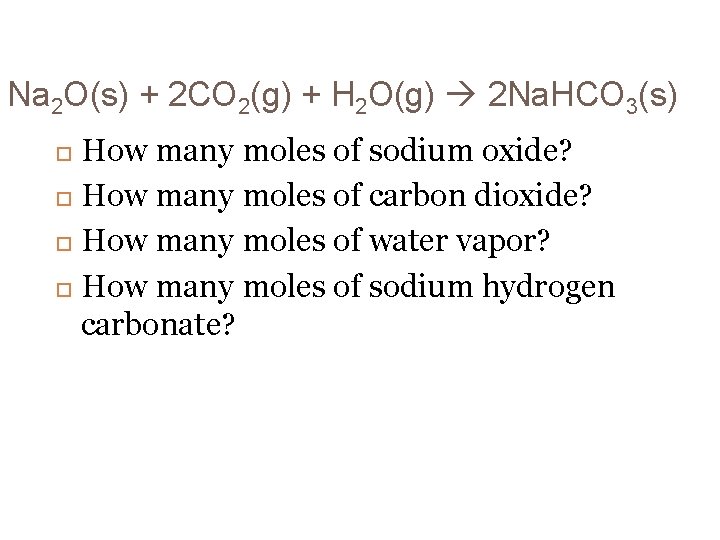

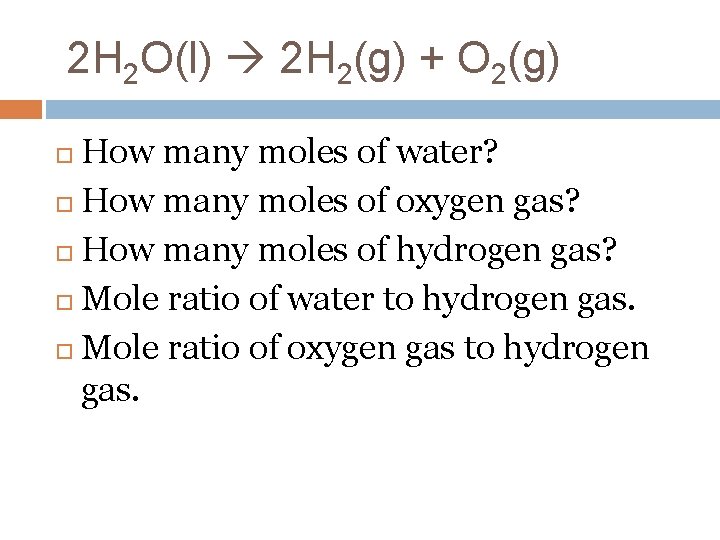
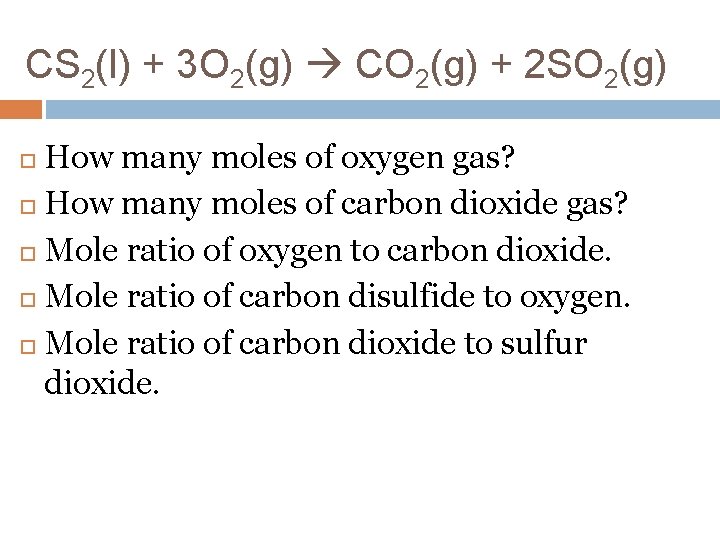
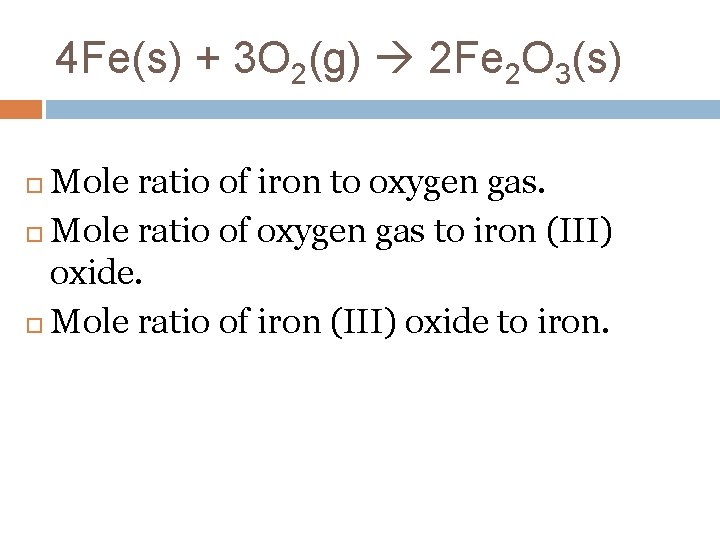



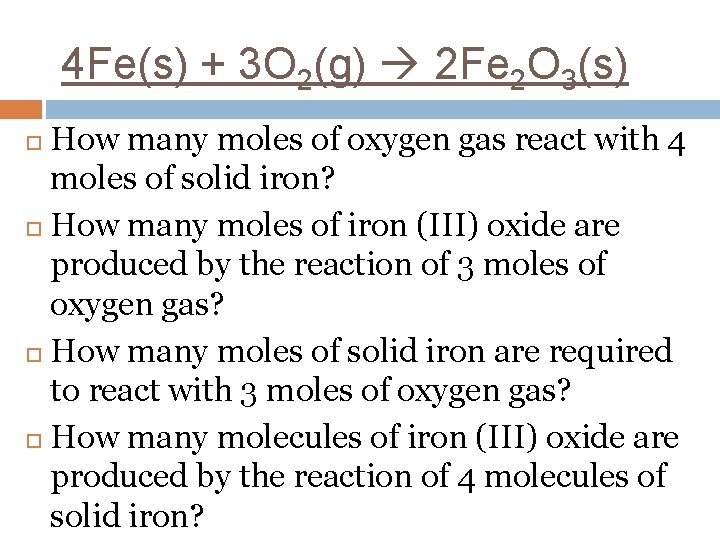
- Slides: 40

Mole relationships in chemical equations

DO-NOW – Silent and Independent! Remember: � (g) = gas (l) = liquid (s) = solid �(aq) = aqueous Must use rules for naming/writing formulas �Ionic compounds – balance charges, NO prefixes �Covalent compounds – use prefixes

Information from Chemical Equations To produce fertilizers, Nitrogen gas is combined with Hydrogen gas to produce Nitrogen trihydride (ammonia). N 2(g) + H 2(g) NH 3(g)

Information from Chemical Equations Making fertilizer uses a lot of energy and a lot of raw materials Efficiency is key!! How can knowledge about chemical reactions make This process as efficient as possible?

The Law of Conservation of Mass In a chemical reaction, matter is never created or destroyed. The number of atoms and the mass of the reactants must be equal to the number of atoms and the mass of the products.

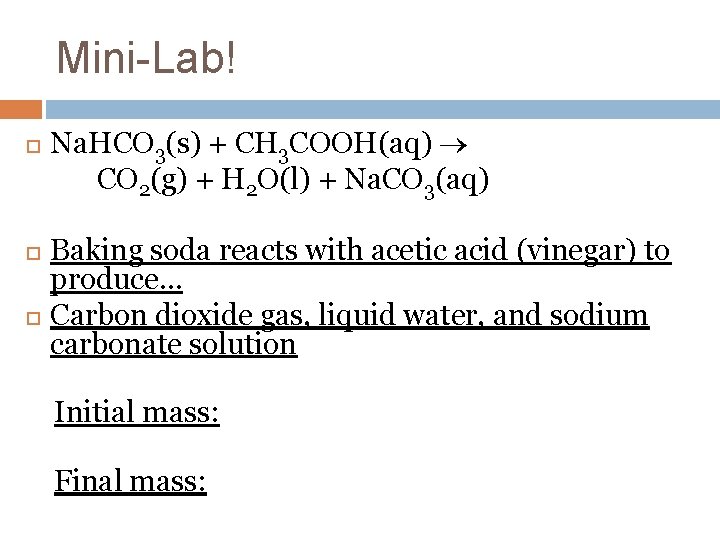
Mini-Lab! Na. HCO 3(s) + CH 3 COOH(aq) CO 2(g) + H 2 O(l) + Na. CO 3(aq) Baking soda reacts with acetic acid (vinegar) to produce… Carbon dioxide gas, liquid water, and sodium carbonate solution Initial mass: Final mass:

Mini - Lab 1. 2. 3. 4. 5. 6. Find the mass of both reagents IN THEIR CONTAINERS. Record this mass as your initial mass Remove materials from balance Carefully pour the acetic acid into the baking soda. Agitate until bubbling stops Find the mass of both reagents IN THEIR CONTAINERS again. Record this mass as your final mass

What were your results? Thoughts? Why doesn’t this reaction seem to obey the law of conservation of mass? What could we do to confirm that it satisfies the law of conservation of mass?

More examples: Law of Conservation of Mass CS 2(l) + O 2(g) CO 2(g) + SO 2(g) If I reacted 5 grams of CS 2 with 5 grams of O 2, and the reaction produced 2 grams of CO 2, how many grams of SO 2 must have been produced? If my products had a total mass of 20 grams, how many grams of reactants must I have started with? If I reacted 3 grams of CS 2 with an unknown number of grams of O 2 and produced a total of 4 grams of products, what was the mass of the O 2?

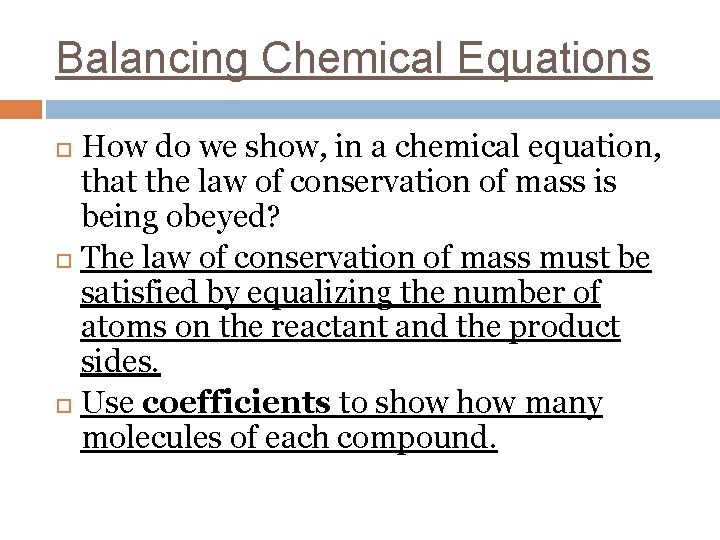
Balancing Chemical Equations How do we show, in a chemical equation, that the law of conservation of mass is being obeyed? The law of conservation of mass must be satisfied by equalizing the number of atoms on the reactant and the product sides. Use coefficients to show many molecules of each compound.

H 2(g) n H 2(g) + F 2(g) HF(g) + F 2(g) 2 HF(g)

Rules for balancing an equation 1. Only change the coefficients that appear in front of an element or compound 2. Never change any subscripts in a formula 3. Coefficients should be written as the lowest possible ratios 4. Begin by balancing elements that appear ONLY once on each side of the equation

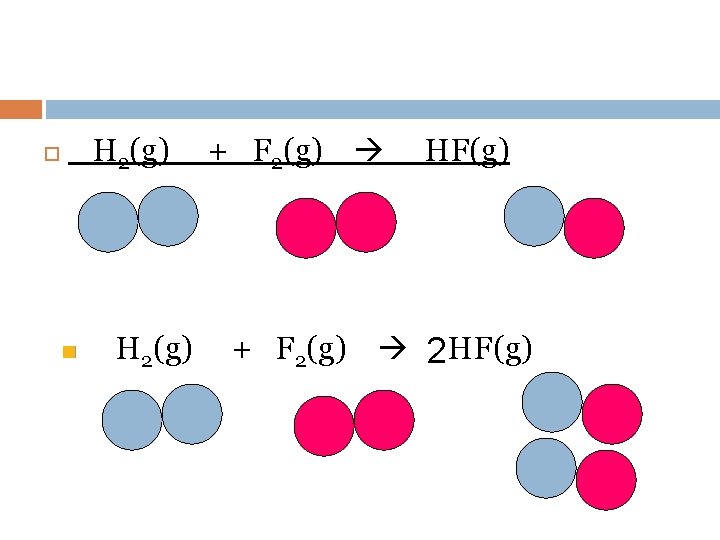
n H 2 O(l) H 2(g) + 2 H 2 O(l) 2 H 2(g) + O 2(g)

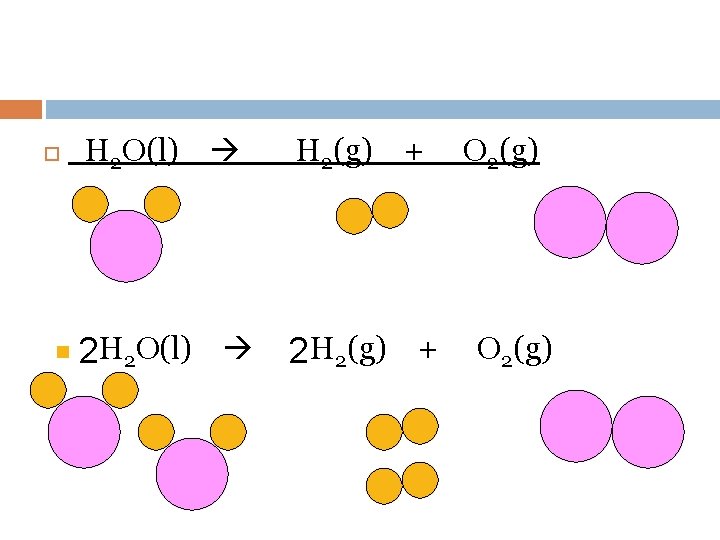
Balancing Chemical Equations Ca(s) + n 8 Ca(s) + S 8(s) Ca. S(s) 8 Ca. S(s)

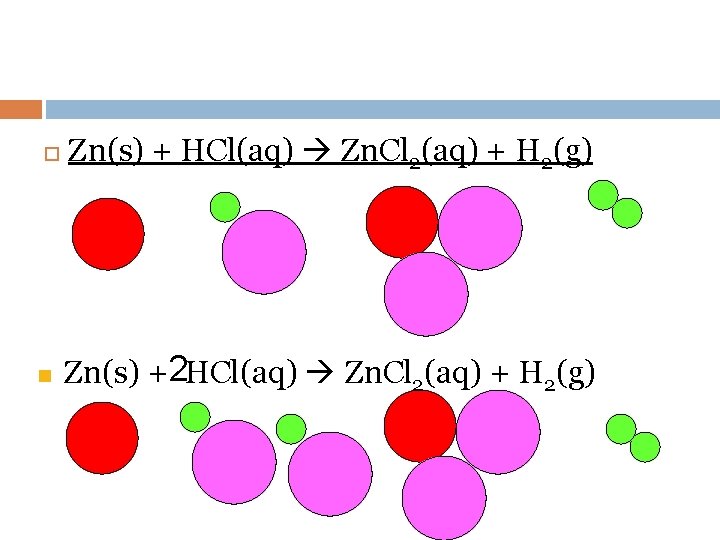
n Zn(s) + HCl(aq) Zn. Cl 2(aq) + H 2(g) Zn(s) +2 HCl(aq) Zn. Cl 2(aq) + H 2(g)

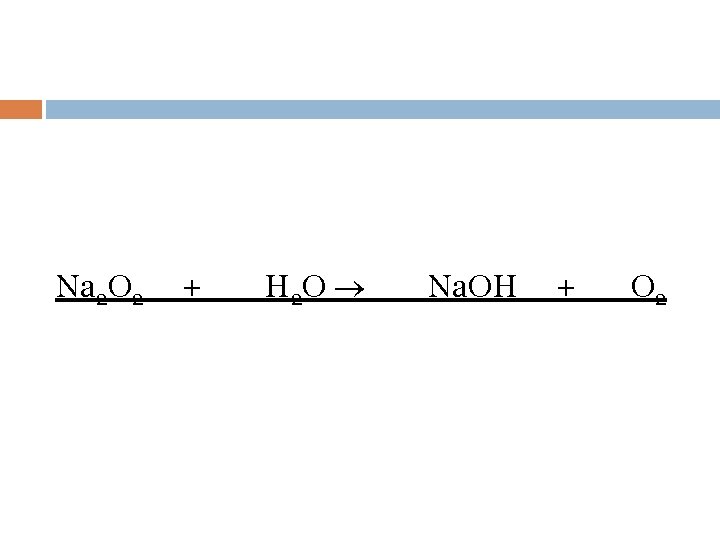
Na 2 O 2 + H 2 O Na. OH + O 2

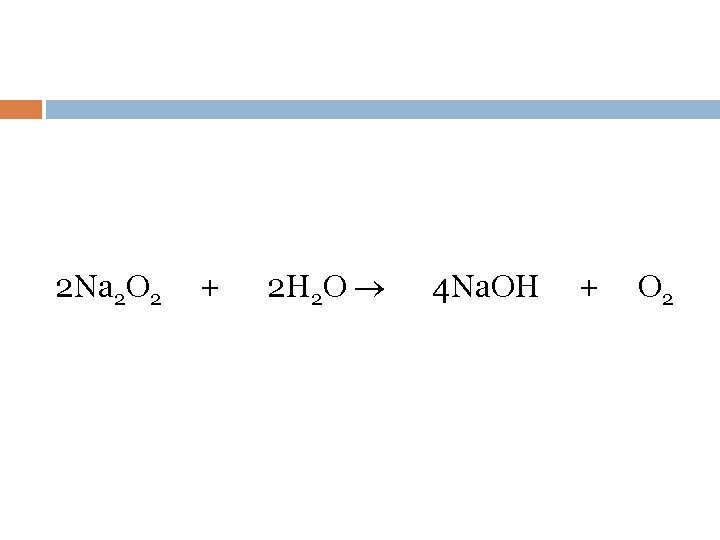
2 Na 2 O 2 + 2 H 2 O 4 Na. OH + O 2

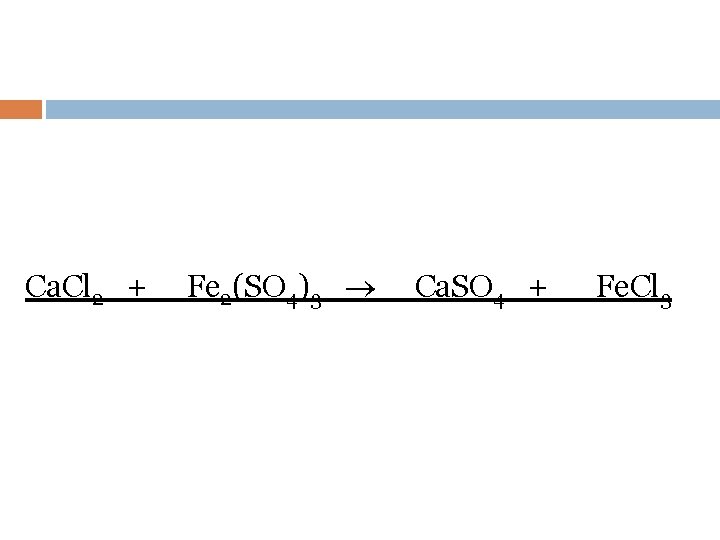
Ca. Cl 2 + Fe 2(SO 4)3 Ca. SO 4 + Fe. Cl 3

3 Ca. Cl 2 + Fe 2(SO 4)3 3 Ca. SO 4 + 2 Fe. Cl 3

Liquid silicon tetrachloride reacts with liquid water to produce solid silicon dioxide and a solution of hydrochloric acid (HCl). Write the chemical equation for this word equation Balance it

Si. Cl 4(l) + 2 H 2 O(l) Si. O 2(s) + 4 HCl(aq)

Mole Ratios in Chemical Equations

Building a Tricycle To build a tricycle, you need a frame, a seat, 3 wheels, a set of handlebars and 2 pedals If I wanted to build 2 tricycles, How many seats would I need? How many wheels? What if I wanted 346 tricycles?

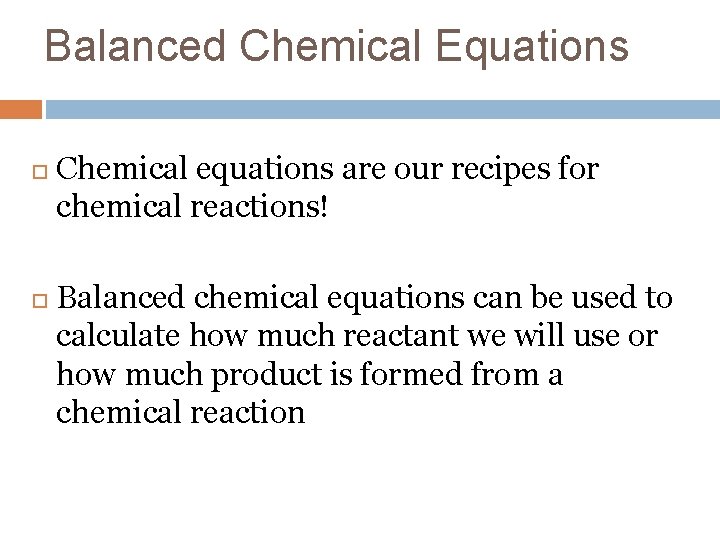
Balanced Chemical Equations Chemical equations are our recipes for chemical reactions! Balanced chemical equations can be used to calculate how much reactant we will use or how much product is formed from a chemical reaction

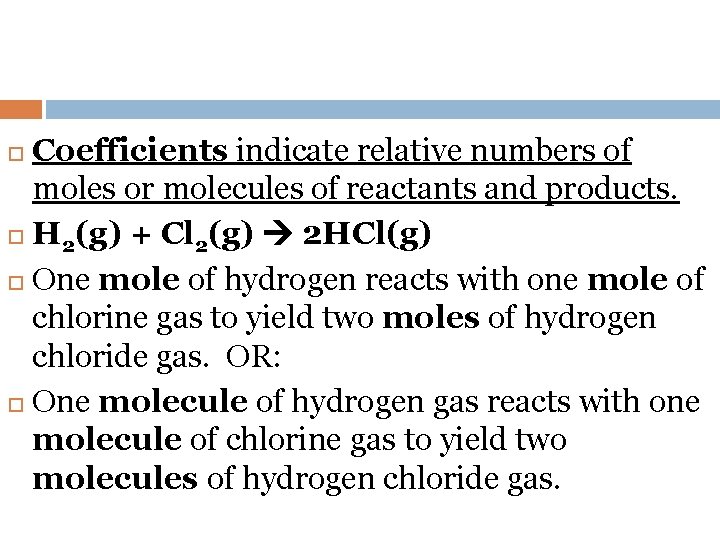
Coefficients indicate relative numbers of moles or molecules of reactants and products. H 2(g) + Cl 2(g) 2 HCl(g) One mole of hydrogen reacts with one mole of chlorine gas to yield two moles of hydrogen chloride gas. OR: One molecule of hydrogen gas reacts with one molecule of chlorine gas to yield two molecules of hydrogen chloride gas.

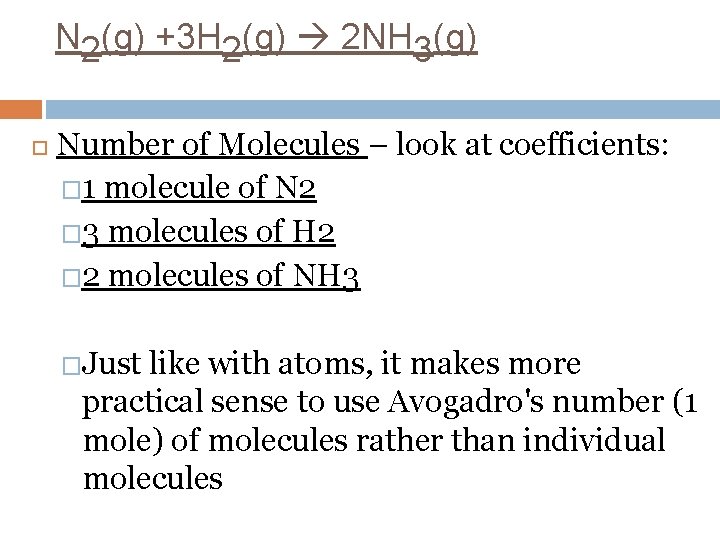
N 2(g) +3 H 2(g) 2 NH 3(g) Number of Molecules – look at coefficients: � 1 molecule of N 2 � 3 molecules of H 2 � 2 molecules of NH 3 �Just like with atoms, it makes more practical sense to use Avogadro's number (1 mole) of molecules rather than individual molecules

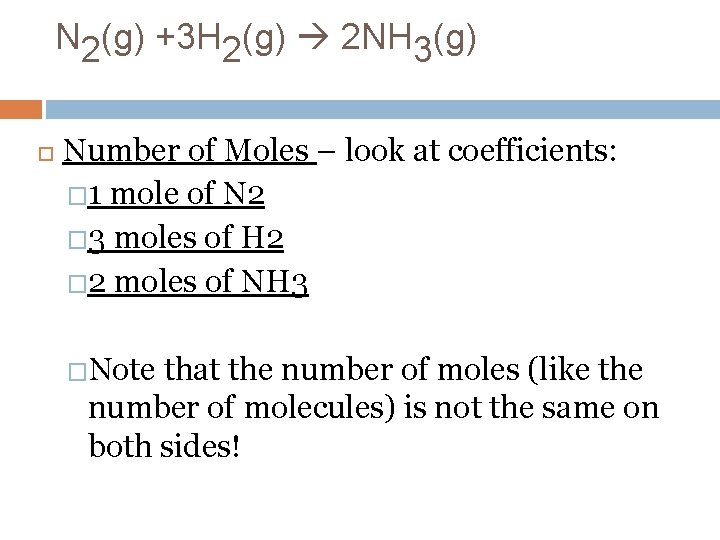
N 2(g) +3 H 2(g) 2 NH 3(g) Number of Moles – look at coefficients: � 1 mole of N 2 � 3 moles of H 2 � 2 moles of NH 3 �Note that the number of moles (like the number of molecules) is not the same on both sides!
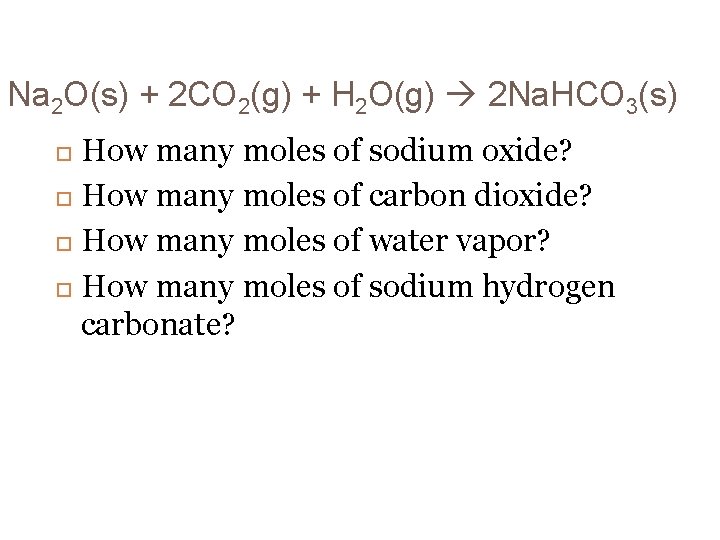
Na 2 O(s) + 2 CO 2(g) + H 2 O(g) 2 Na. HCO 3(s) How many moles of sodium oxide? How many moles of carbon dioxide? How many moles of water vapor? How many moles of sodium hydrogen carbonate?


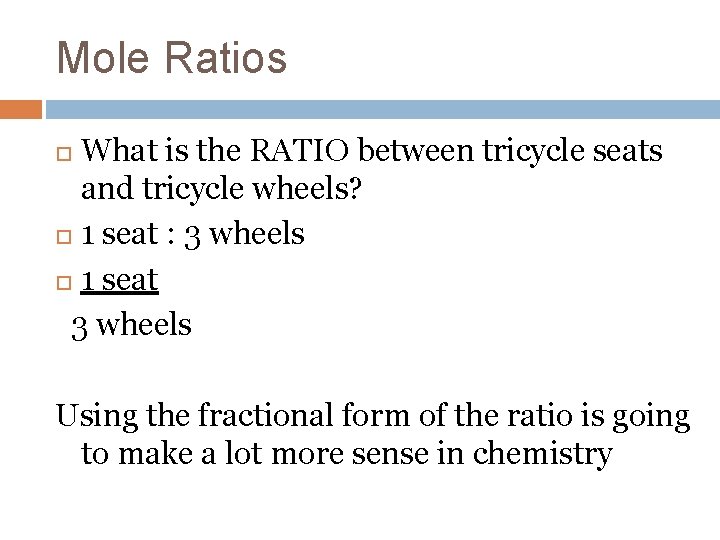
Mole Ratios What is the RATIO between tricycle seats and tricycle wheels? 1 seat : 3 wheels 1 seat 3 wheels Using the fractional form of the ratio is going to make a lot more sense in chemistry

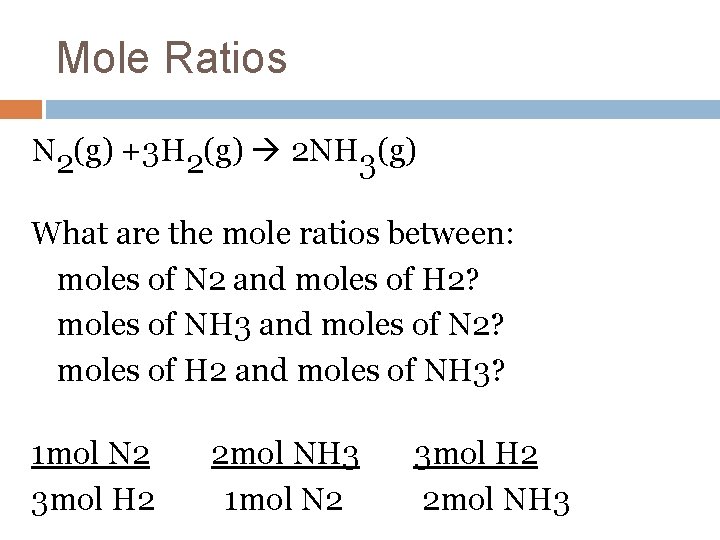
Mole Ratios N 2(g) +3 H 2(g) 2 NH 3(g) What are the mole ratios between: moles of N 2 and moles of H 2? moles of NH 3 and moles of N 2? moles of H 2 and moles of NH 3? 1 mol N 2 3 mol H 2 2 mol NH 3
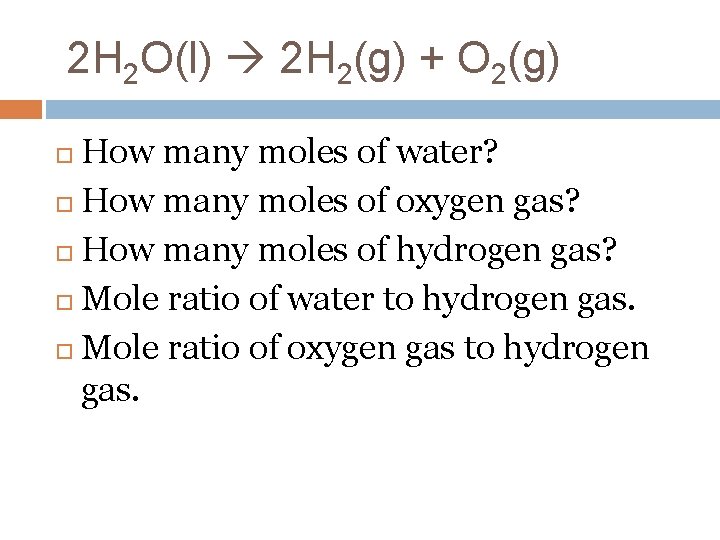
2 H 2 O(l) 2 H 2(g) + O 2(g) How many moles of water? How many moles of oxygen gas? How many moles of hydrogen gas? Mole ratio of water to hydrogen gas. Mole ratio of oxygen gas to hydrogen gas.
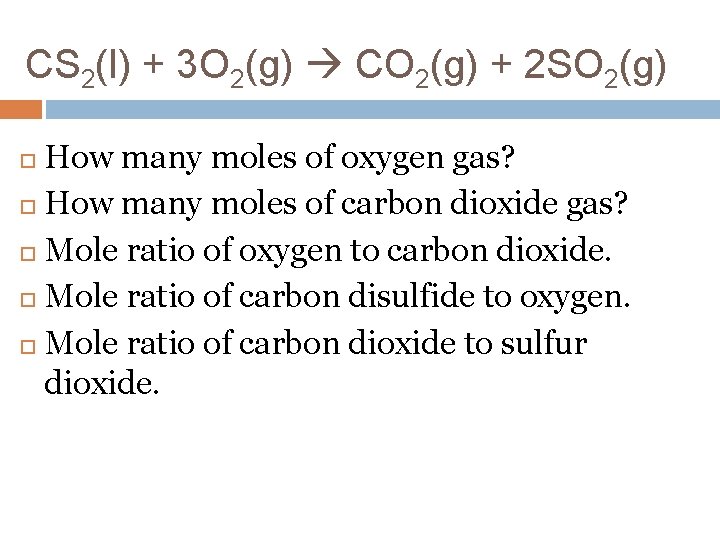
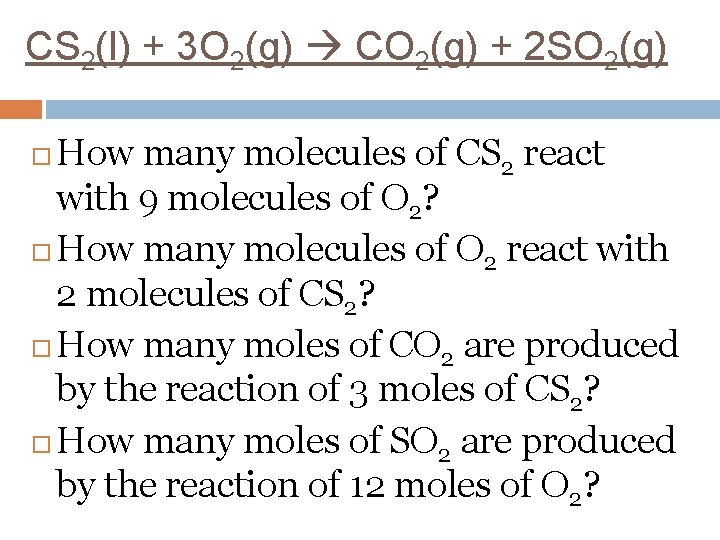
CS 2(l) + 3 O 2(g) CO 2(g) + 2 SO 2(g) How many moles of oxygen gas? How many moles of carbon dioxide gas? Mole ratio of oxygen to carbon dioxide. Mole ratio of carbon disulfide to oxygen. Mole ratio of carbon dioxide to sulfur dioxide.
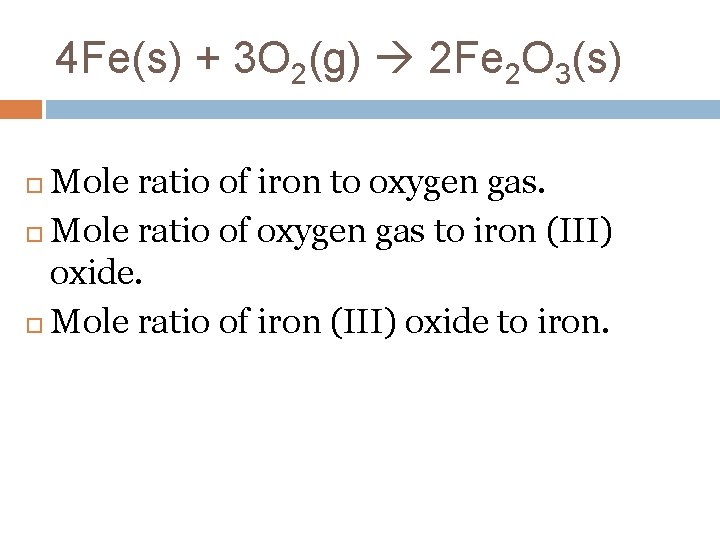
4 Fe(s) + 3 O 2(g) 2 Fe 2 O 3(s) Mole ratio of iron to oxygen gas. Mole ratio of oxygen gas to iron (III) oxide. Mole ratio of iron (III) oxide to iron.


N 2(g) +3 H 2(g) 2 NH 3(g) How many moles of ammonia will I get by reacting 2 moles of nitrogen gas? GIVEN MOLE RATIO 2 mol N 2 x 2 mol NH 3 1 1 mol N 2 = 4 mol NH 3 The ONLY way to relate amounts of two different substances is to use the MOLE RATIO!!

MOLE conversions To get from moles of substance A to moles of substance B, always use the mole ratio! STEP 1: Write the given value (with a unit) over 1 STEP 2: Write a blank conversion factor STEP 3: Fill in the units to cancel your given unit STEP 4: Complete the mole ratio STEP 5: Multiply/Divide to get final answer

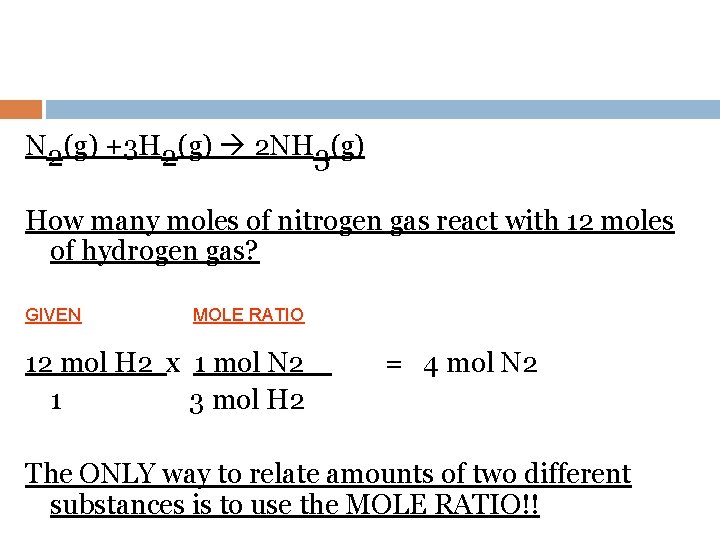
N 2(g) +3 H 2(g) 2 NH 3(g) How many moles of nitrogen gas react with 12 moles of hydrogen gas? GIVEN MOLE RATIO 12 mol H 2 x 1 mol N 2 1 3 mol H 2 = 4 mol N 2 The ONLY way to relate amounts of two different substances is to use the MOLE RATIO!!
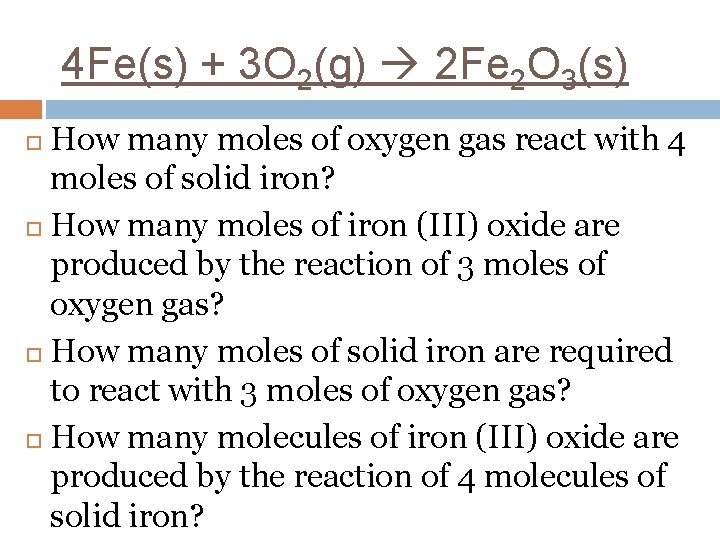
4 Fe(s) + 3 O 2(g) 2 Fe 2 O 3(s) How many moles of oxygen gas react with 4 moles of solid iron? How many moles of iron (III) oxide are produced by the reaction of 3 moles of oxygen gas? How many moles of solid iron are required to react with 3 moles of oxygen gas? How many molecules of iron (III) oxide are produced by the reaction of 4 molecules of solid iron?

CS 2(l) + 3 O 2(g) CO 2(g) + 2 SO 2(g) How many molecules of CS 2 react with 9 molecules of O 2? How many molecules of O 2 react with 2 molecules of CS 2? How many moles of CO 2 are produced by the reaction of 3 moles of CS 2? How many moles of SO 2 are produced by the reaction of 12 moles of O 2?
 Mole relationships in chemical equations
Mole relationships in chemical equations Mole mass and mole volume relationships
Mole mass and mole volume relationships The way my mother speaks
The way my mother speaks Quantitative relationships in chemical equations
Quantitative relationships in chemical equations Stoichiometry: mole-mole problems
Stoichiometry: mole-mole problems Mole-mole factor
Mole-mole factor Stoichiometry worksheet #2 (mole-mass mass-mole problems)
Stoichiometry worksheet #2 (mole-mass mass-mole problems) Molecular mass of sucrose
Molecular mass of sucrose Stoichiometry mole-mole
Stoichiometry mole-mole Mass to grams conversion
Mass to grams conversion Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Translating chemical equations
Translating chemical equations Unit chemical quantities the mole 1 step
Unit chemical quantities the mole 1 step Stoichiometry island diagram
Stoichiometry island diagram Mass relationships in chemical reactions
Mass relationships in chemical reactions Chapter 7 chemical formulas and chemical compounds test
Chapter 7 chemical formulas and chemical compounds test Chemical formulas and chemical compounds chapter 7 review
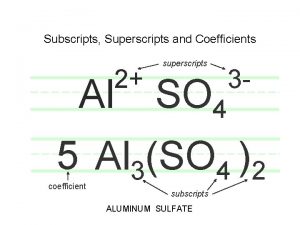
Chemical formulas and chemical compounds chapter 7 review Subscript for aluminum
Subscript for aluminum Chemistry chapter 8 review chemical equations and reactions
Chemistry chapter 8 review chemical equations and reactions Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chemical equations and reactions chapter 8
Chemical equations and reactions chapter 8 Balancing chemical equations worksheet
Balancing chemical equations worksheet I intro
I intro Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Writing and balancing chemical equations worksheet
Writing and balancing chemical equations worksheet Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes The calculation of quantities in chemical equations
The calculation of quantities in chemical equations Toxic reactions chemical equations answer key
Toxic reactions chemical equations answer key Kahoot balancing chemical equations
Kahoot balancing chemical equations Chemical reactions rearranging atoms worksheet answers
Chemical reactions rearranging atoms worksheet answers Balancing equations counting atoms
Balancing equations counting atoms How to balance a chemical equation
How to balance a chemical equation Balancing equations vocabulary
Balancing equations vocabulary Balancing equations vocabulary
Balancing equations vocabulary Decomposition reactions
Decomposition reactions Chemical equations definition
Chemical equations definition Chemical equation of phosphorus
Chemical equation of phosphorus Is it balanced
Is it balanced Balancing equations pogil
Balancing equations pogil