The Mole 466 The Mole A mole is







- Slides: 9

The Mole (466)

The Mole • A mole is a number (like a dozen means 12). • Having this number of atoms allows us to easily convert atomic masses to molar masses. • The abbreviation for 1 mole is 1 mol • 6. 02 x 1023 is a “mole” or “Avogadro’s number” 1 mol = 6. 02 x 1023 molecules (or atoms)

Molar mass • The mass of one mole is called “molar mass” • E. g. 1 mol Li = 6. 94 g/mol of Li • (Use your periodic table) What are the following molar masses? S 32. 06 g/mol SO 2 64. 06 g/mol Cu 3(BO 3)2 308. 27 g/mol Cu x 3 = 63. 55 x 3 = 190. 65 B x 2 = 10. 81 x 2 = 21. 62 O x 6 = 16. 00 x 6 = 96. 00 308. 27

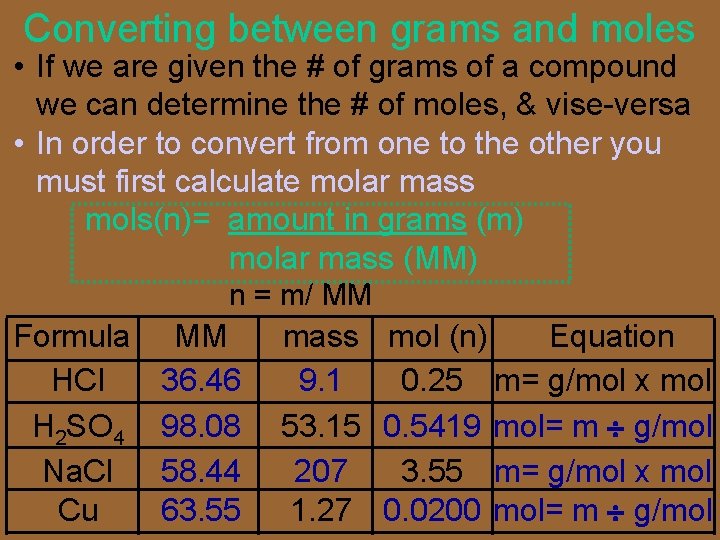
Converting between grams and moles • If we are given the # of grams of a compound we can determine the # of moles, & vise-versa • In order to convert from one to the other you must first calculate molar mass mols(n)= amount in grams (m) molar mass (MM) n = m/ MM Formula HCl H 2 SO 4 Na. Cl Cu MM 36. 46 98. 08 58. 44 63. 55 mass mol (n) Equation 9. 1 0. 25 m= g/mol x mol 53. 15 0. 5419 mol= m g/mol 207 3. 55 m= g/mol x mol 1. 27 0. 0200 mol= m g/mol

• Watermelons stored at room temperature (21 degrees Celsius) are richer in nutrients than refrigerated ones. • By letting your watermelons ripen outside the fridge, you are also allowing them to gain lycopene and beta-carotene, both of which are important nutrients. • Lycopene, an antioxidant, has been linked to reducing the risk of several diseases such as prostate cancer, and lowering inflammation that may cause hypertension and heart disease.

Making Molar Solutions From Solids 466

What are molar solutions? A molar solution is one that expresses “concentration” in moles per volume Molarity = # of mols volume of solution(L) M=n V Usually the units of M are in mol/L L refers to entire volume, not just the water!

Calculations with molar solutions Q: How many moles of Na. Cl are required to make 7. 5 L of a 0. 10 M solution? M= n/V # mol Na. Cl = 7. 5 L x 0. 10 mol Na. Cl = 0. 75 mol 1 L

Practice Problems 1. How many grams of nitric acid are present in 1. 0 L of a 1. 0 M HNO 3 solution? 63 g 2. Calculate the # of grams needed to produce each: a) 22 g a) 0. 20 L of 1. 5 M KCl b) 0. 160 L of 0. 300 M HCl b) 1. 75 g 3. Give the molarity of a solution containing 10 g of each solute in 2. 5 L of solution: a)H 2 SO 4 a) 0. 041 mol/L b)Ca(OH)2 b) 0. 054 mol/L