the Mole What is a mole A mole




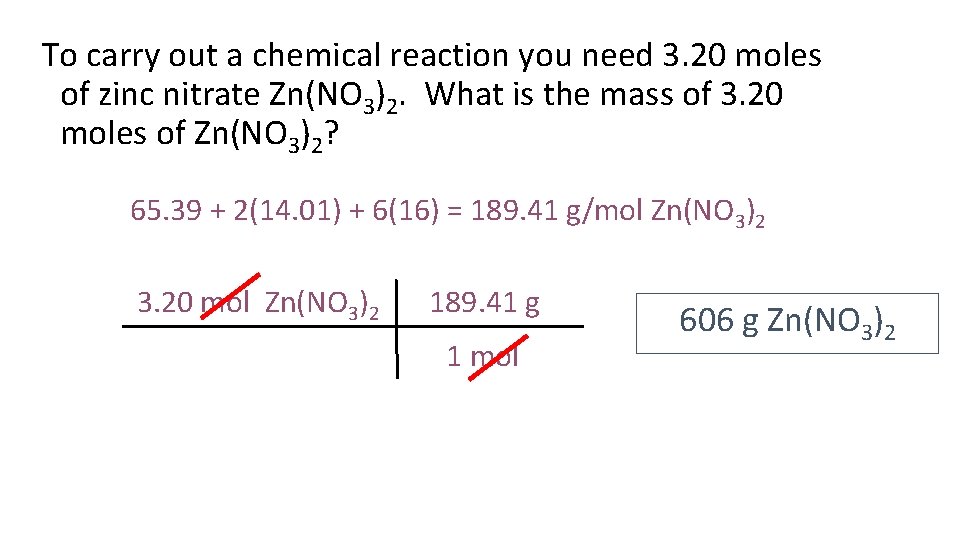

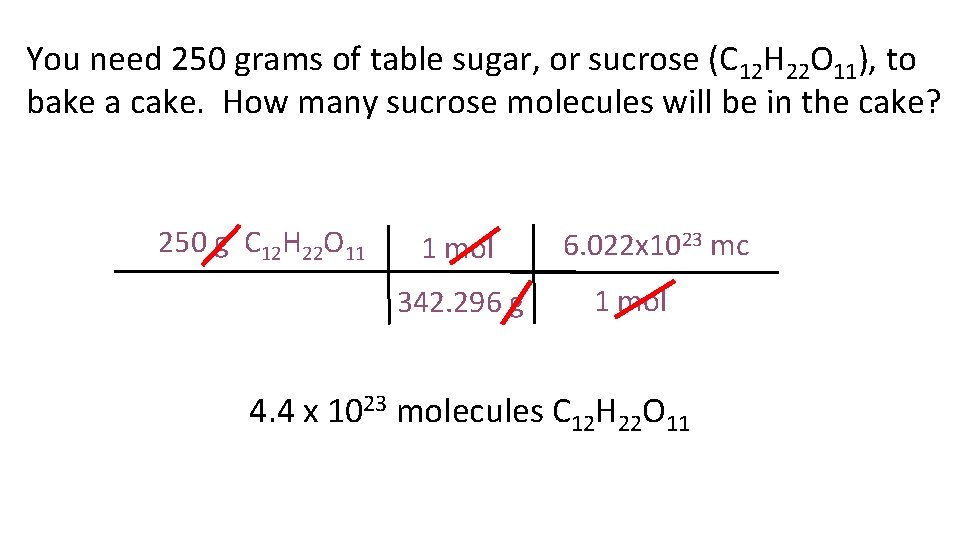
- Slides: 17

the Mole

What is a mole? • A mole is the SI unit used to measure the amount of a substance • It is used to measure LARGE amounts of very small things – like atoms, molecules, formula units, etc. • Abbreviation = mol

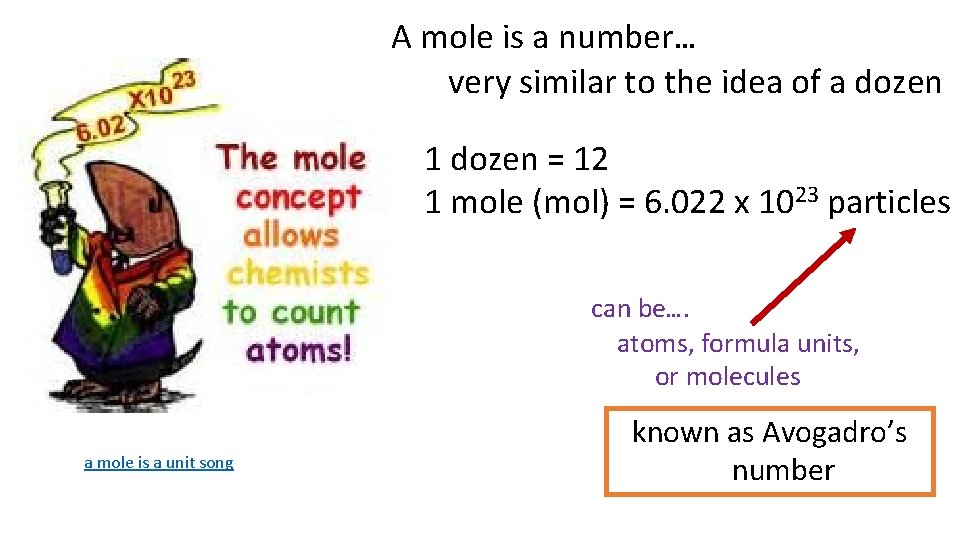
A mole is a number… very similar to the idea of a dozen 1 dozen = 12 1 mole (mol) = 6. 022 x 1023 particles can be…. atoms, formula units, or molecules a mole is a unit song known as Avogadro’s number


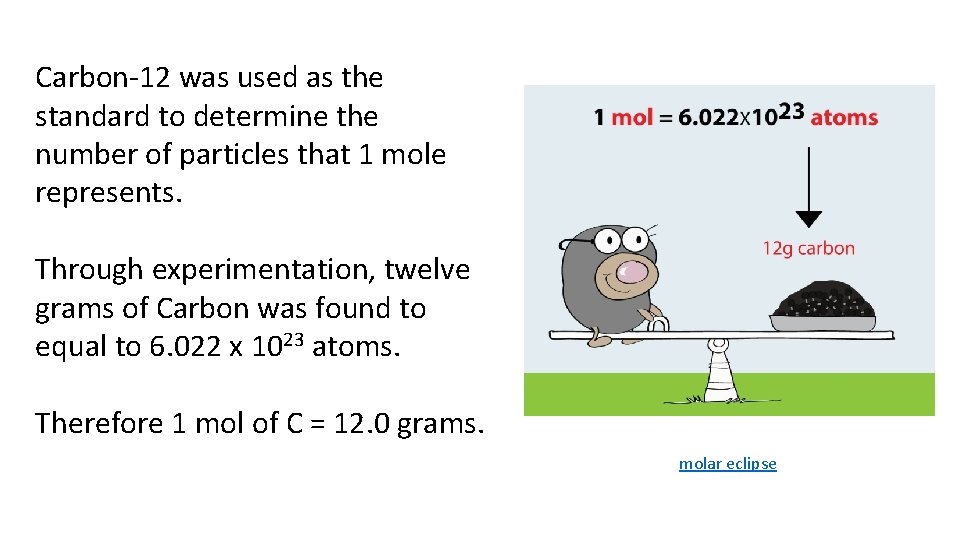
Carbon-12 was used as the standard to determine the number of particles that 1 mole represents. Through experimentation, twelve grams of Carbon was found to equal to 6. 022 x 1023 atoms. Therefore 1 mol of C = 12. 0 grams. molar eclipse

Molar mass the mass of 1 mole of a substance in grams • Molar mass of an element (in grams) = 1 mole • Molar mass of compound (in grams) = 1 mole 1 mol = molar mass

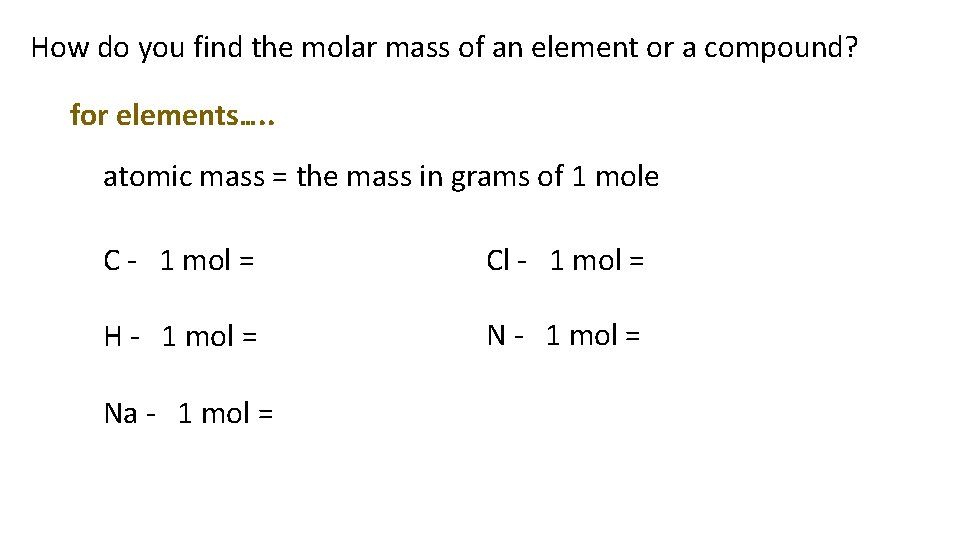
How do you find the molar mass of an element or a compound? for elements…. . atomic mass = the mass in grams of 1 mole C - 1 mol = Cl - 1 mol = H - 1 mol = Na - 1 mol =

for compounds…. . Complete an atom inventory and multiply each by the atomic mass. Add all the masses together. Mg. Cl 2 – 24. 31 + 2(35. 45) = 95. 21 g/mol 1 mol = 95. 21 g Ca(OH)2 - 40. 08 + 2(16) + 2(1. 008) = 74. 096 g/mol 1 mol = 74. 096 g C 6 H 12 O 6 - 6(12. 01) + 12(1. 008) + 6(16) = 180. 156 g/mol 1 mol = 180. 156 g

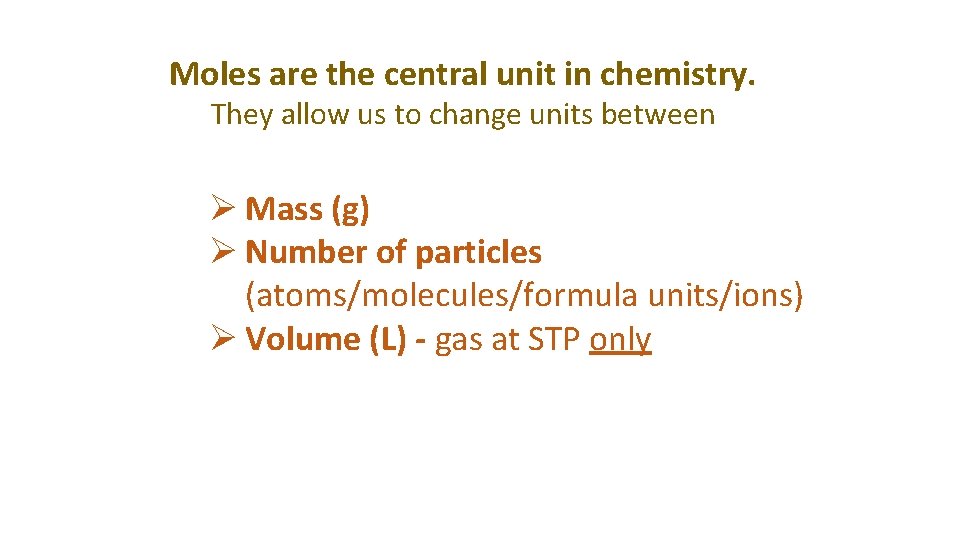
Moles are the central unit in chemistry. They allow us to change units between Ø Mass (g) Ø Number of particles (atoms/molecules/formula units/ions) Ø Volume (L) - gas at STP only

Moles and Mass The conversion factor is 1 mol = Molar Mass Convert 5. 0 moles of Sulfur to grams 160 g S
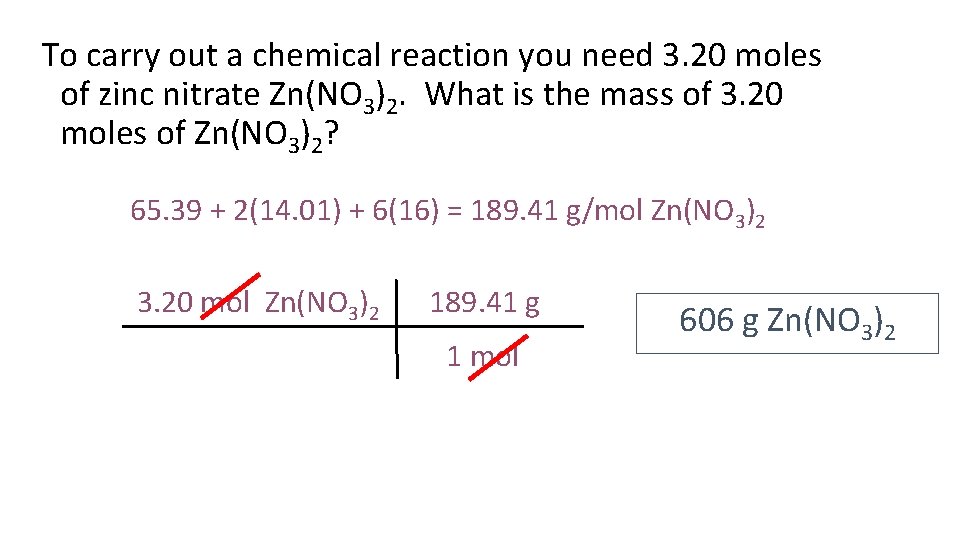
To carry out a chemical reaction you need 3. 20 moles of zinc nitrate Zn(NO 3)2. What is the mass of 3. 20 moles of Zn(NO 3)2? 65. 39 + 2(14. 01) + 6(16) = 189. 41 g/mol Zn(NO 3)2 3. 20 mol Zn(NO 3)2 189. 41 g 1 mol 606 g Zn(NO 3)2

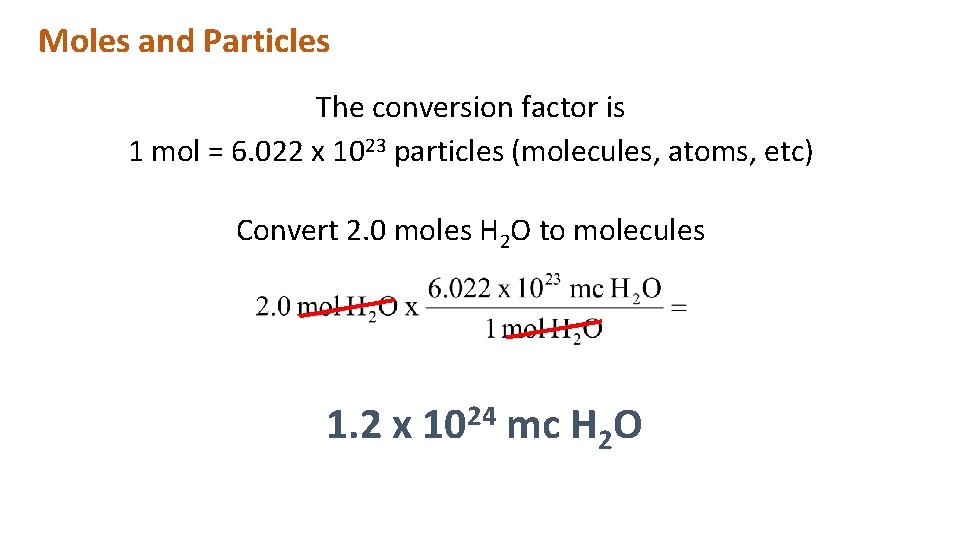
Moles and Particles The conversion factor is 1 mol = 6. 022 x 1023 particles (molecules, atoms, etc) Convert 2. 0 moles H 2 O to molecules 1. 2 x 1024 mc H 2 O

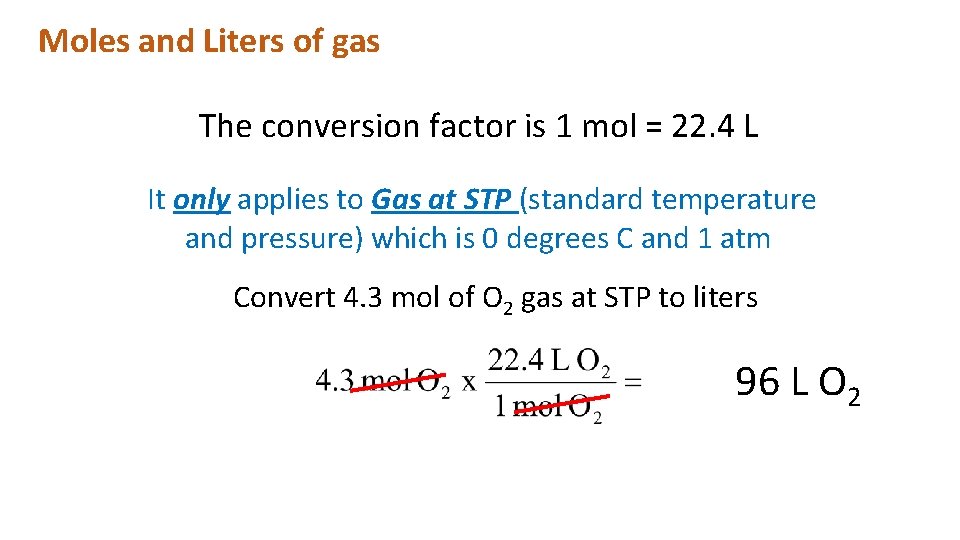
Moles and Liters of gas The conversion factor is 1 mol = 22. 4 L It only applies to Gas at STP (standard temperature and pressure) which is 0 degrees C and 1 atm Convert 4. 3 mol of O 2 gas at STP to liters 96 L O 2

A piece of marble contains 8. 74 x 1023 formula units of calcium carbonate. How many moles of Ca. CO 3 is that? 8. 74 x 1023 fo un Ca. CO 3 1 mol 6. 022 x 1023 fo un 1. 45 mol Ca. CO 3

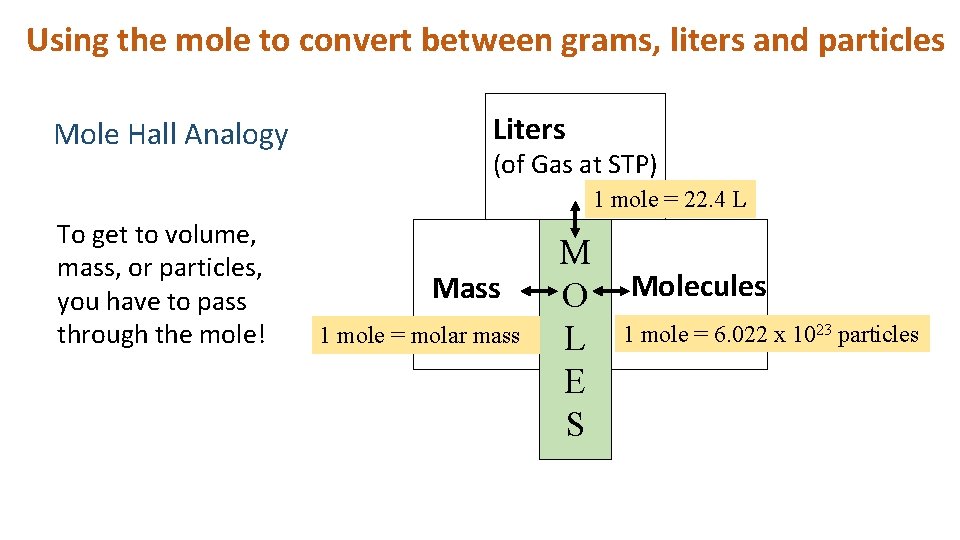
Using the mole to convert between grams, liters and particles Mole Hall Analogy Liters (of Gas at STP) 1 mole = 22. 4 L To get to volume, mass, or particles, you have to pass through the mole! Mass 1 mole = molar mass M O L E S Molecules 1 mole = 6. 022 x 1023 particles
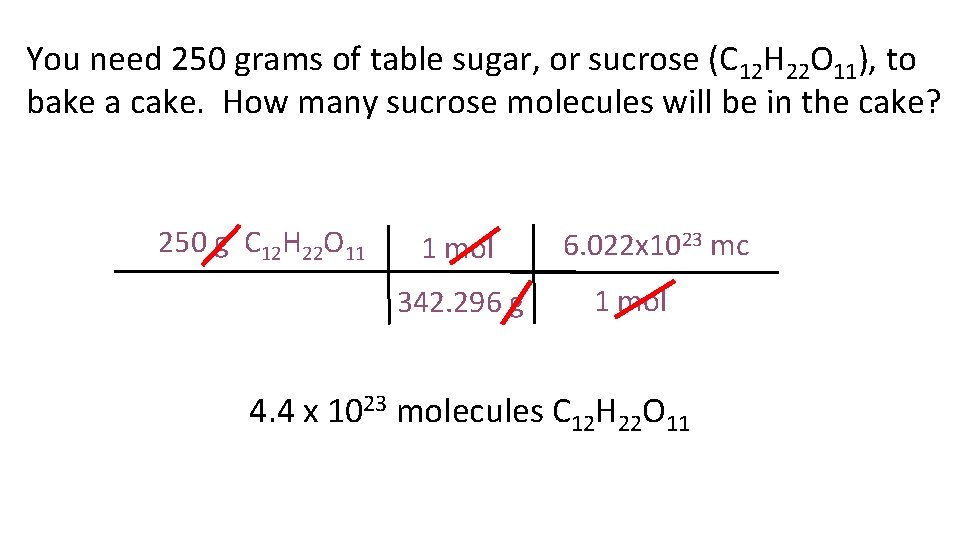
You need 250 grams of table sugar, or sucrose (C 12 H 22 O 11), to bake a cake. How many sucrose molecules will be in the cake? 250 g C 12 H 22 O 11 1 mol 342. 296 g 6. 022 x 1023 mc 1 mol 4. 4 x 1023 molecules C 12 H 22 O 11

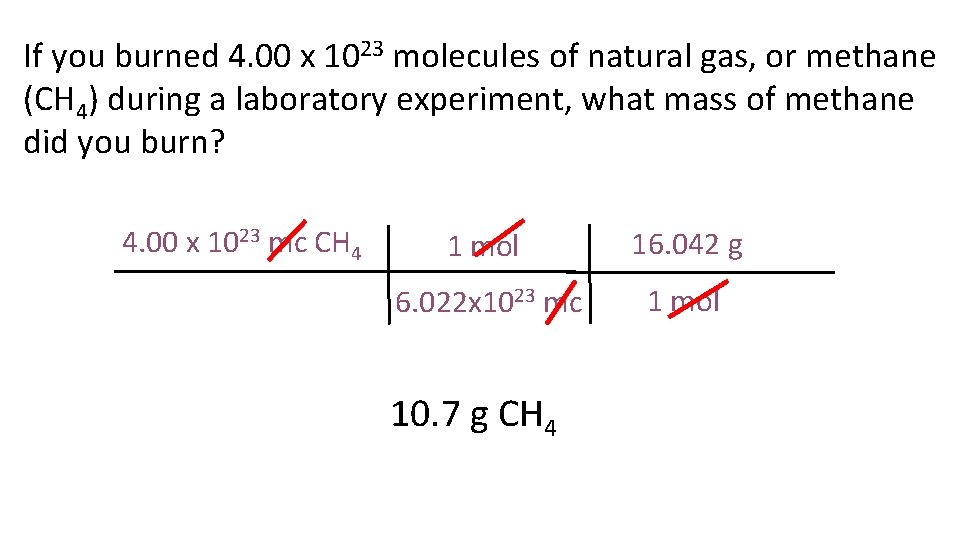
If you burned 4. 00 x 1023 molecules of natural gas, or methane (CH 4) during a laboratory experiment, what mass of methane did you burn? 4. 00 x 1023 mc CH 4 1 mol 16. 042 g 6. 022 x 1023 mc 1 mol 10. 7 g CH 4