Chapter 7 Chemical Quantities The Mole Collections of
















- Slides: 47

Chapter 7 Chemical Quantities The Mole Collections of items include dozen, gross, and mole. 1 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Collection Terms A collection term states a specific number of items. • 1 dozen donuts = 12 donuts • 1 ream of paper = 500 sheets • 1 case = 24 cans Collections of items include dozen, gross, and mole. 2 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

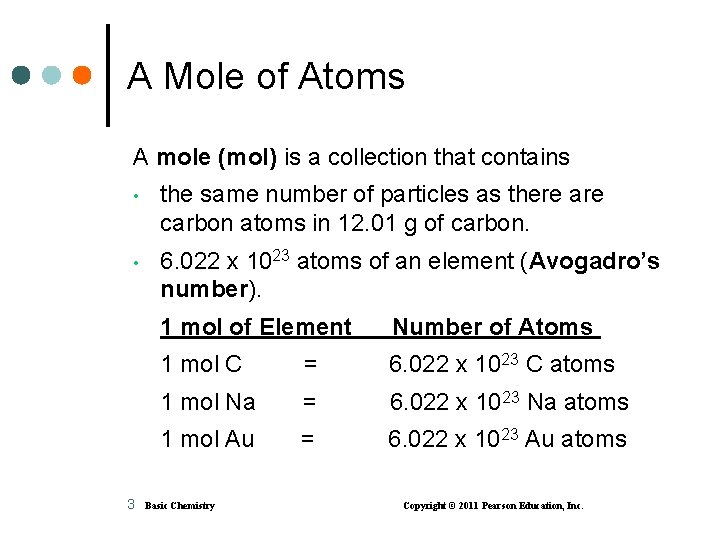
A Mole of Atoms A mole (mol) is a collection that contains • the same number of particles as there are carbon atoms in 12. 01 g of carbon. • 6. 022 x 1023 atoms of an element (Avogadro’s number). 3 1 mol of Element Number of Atoms 1 mol C = 6. 022 x 1023 C atoms 1 mol Na = 6. 022 x 1023 Na atoms 1 mol Au = 6. 022 x 1023 Au atoms Basic Chemistry Copyright © 2011 Pearson Education, Inc.

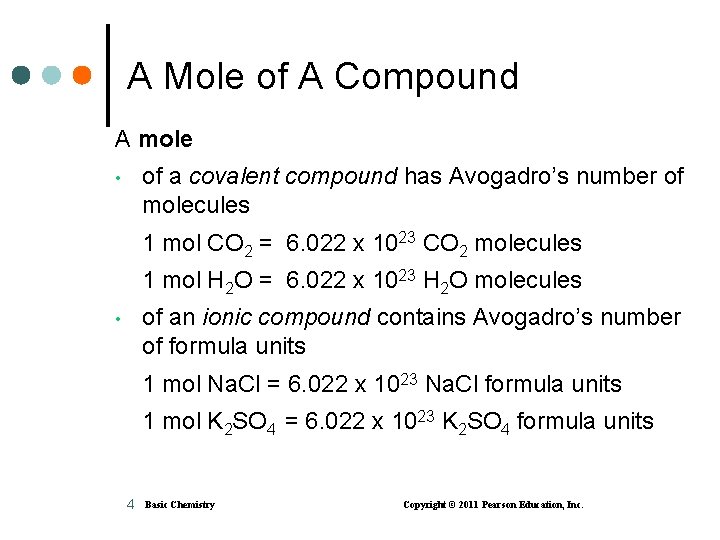
A Mole of A Compound A mole of a covalent compound has Avogadro’s number of molecules • 1 mol CO 2 = 6. 022 x 1023 CO 2 molecules 1 mol H 2 O = 6. 022 x 1023 H 2 O molecules of an ionic compound contains Avogadro’s number of formula units • 1 mol Na. Cl = 6. 022 x 1023 Na. Cl formula units 1 mol K 2 SO 4 = 6. 022 x 1023 K 2 SO 4 formula units 4 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

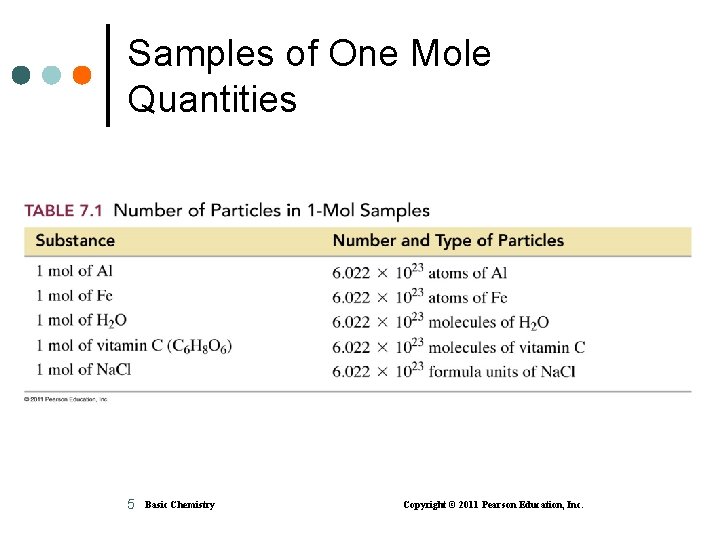
Samples of One Mole Quantities 5 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

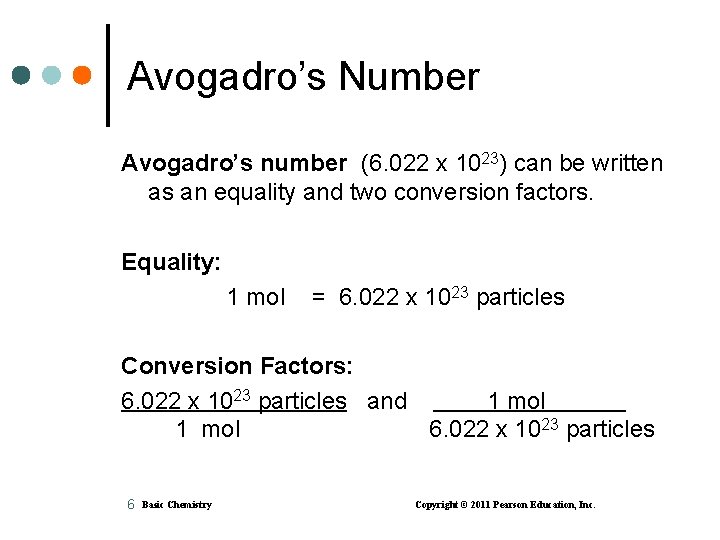
Avogadro’s Number Avogadro’s number (6. 022 x 1023) can be written as an equality and two conversion factors. Equality: 1 mol = 6. 022 x 1023 particles Conversion Factors: 6. 022 x 1023 particles and 1 mol 6. 022 x 1023 particles 6 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Using Avogadro’s Number Avogadro’s number converts moles of a substance to the number of particles. How many Cu atoms are in 0. 50 mol of Cu? 0. 50 mol Cu x 6. 022 x 1023 Cu atoms 1 mol Cu = 3. 0 x 1023 Cu atoms 7 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

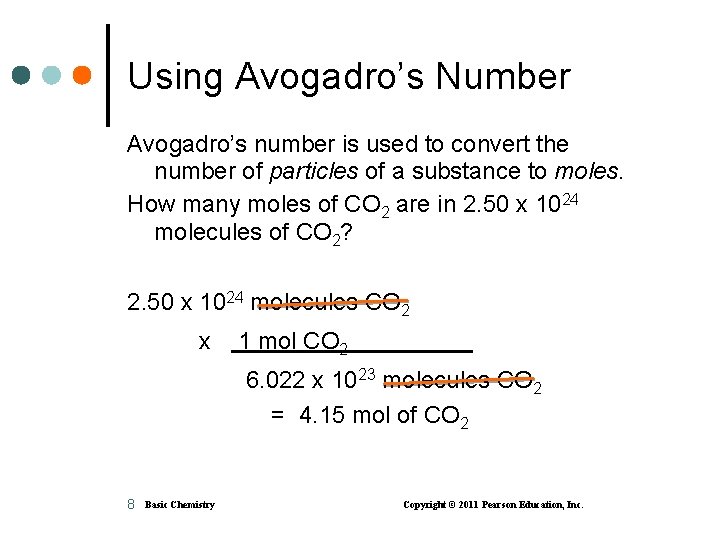
Using Avogadro’s Number Avogadro’s number is used to convert the number of particles of a substance to moles. How many moles of CO 2 are in 2. 50 x 1024 molecules of CO 2? 2. 50 x 1024 molecules CO 2 x 1 mol CO 2 6. 022 x 1023 molecules CO 2 = 4. 15 mol of CO 2 8 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

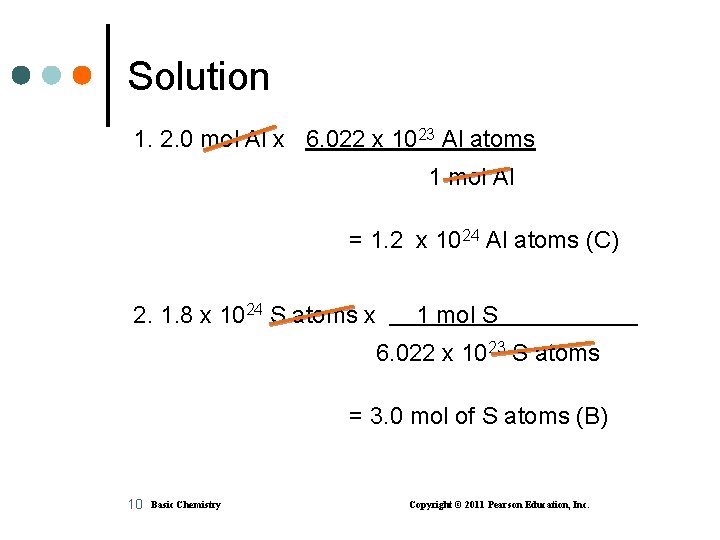
Learning Check 1. The number of atoms in 2. 0 mol of Al is A. 2. 0 Al atoms B. 3. 0 x 1023 Al atoms C. 1. 2 x 1024 Al atoms 2. The number of moles of S in 1. 8 x 1024 atoms of S is A. 1. 0 mol of S atoms B. 3. 0 mol of S atoms C. 1. 1 x 1048 mol of S atoms 9 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Solution 1. 2. 0 mol Al x 6. 022 x 1023 Al atoms 1 mol Al = 1. 2 x 1024 Al atoms (C) 2. 1. 8 x 1024 S atoms x 1 mol S 6. 022 x 1023 S atoms = 3. 0 mol of S atoms (B) 10 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

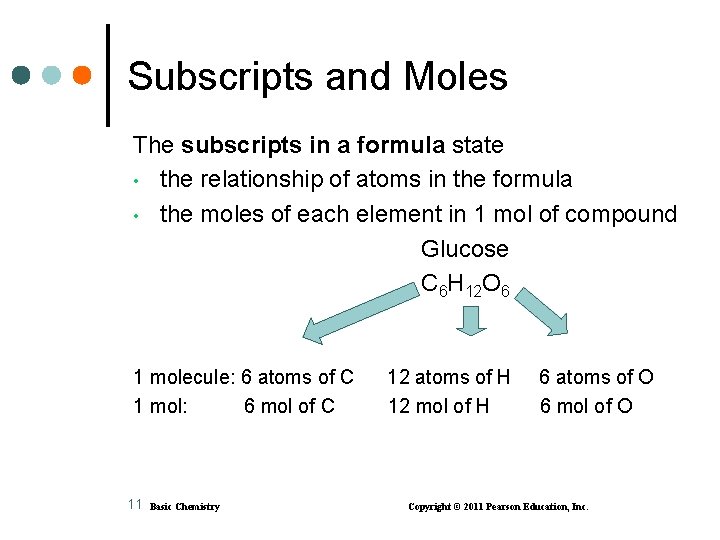
Subscripts and Moles The subscripts in a formula state • the relationship of atoms in the formula • the moles of each element in 1 mol of compound Glucose C 6 H 12 O 6 1 molecule: 6 atoms of C 1 mol: 6 mol of C 11 Basic Chemistry 12 atoms of H 12 mol of H 6 atoms of O 6 mol of O Copyright © 2011 Pearson Education, Inc.

Subscripts State Atoms and Moles 12 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

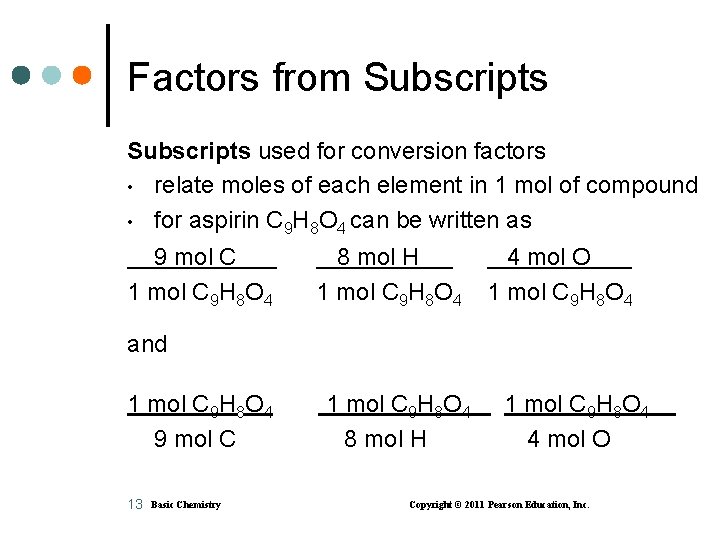
Factors from Subscripts used for conversion factors • relate moles of each element in 1 mol of compound • for aspirin C 9 H 8 O 4 can be written as 9 mol C 1 mol C 9 H 8 O 4 8 mol H 1 mol C 9 H 8 O 4 4 mol O 1 mol C 9 H 8 O 4 and 1 mol C 9 H 8 O 4 9 mol C 13 Basic Chemistry 1 mol C 9 H 8 O 4 8 mol H 1 mol C 9 H 8 O 4 4 mol O Copyright © 2011 Pearson Education, Inc.

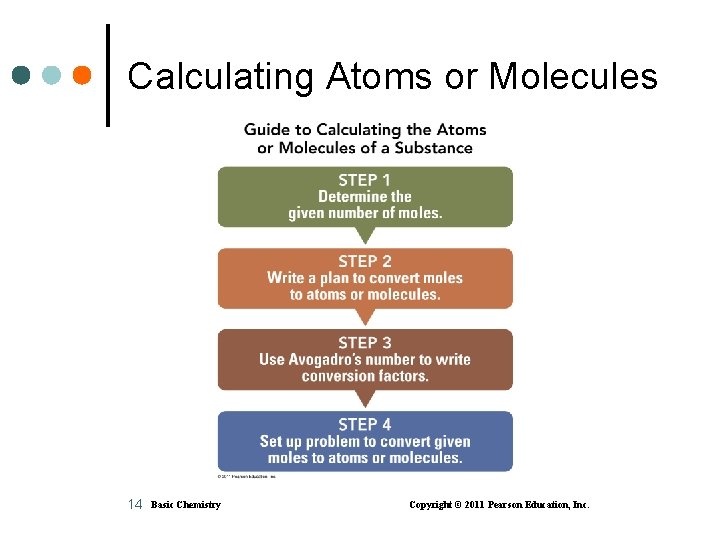
Calculating Atoms or Molecules 14 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

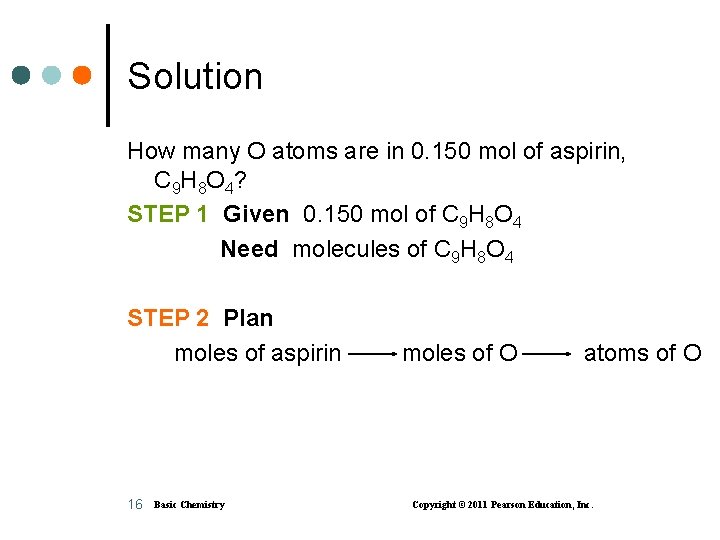
Learning Check How many O atoms are in 0. 150 mol of aspirin, C 9 H 8 O 4? 15 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Solution How many O atoms are in 0. 150 mol of aspirin, C 9 H 8 O 4? STEP 1 Given 0. 150 mol of C 9 H 8 O 4 Need molecules of C 9 H 8 O 4 STEP 2 Plan moles of aspirin 16 Basic Chemistry moles of O atoms of O Copyright © 2011 Pearson Education, Inc.

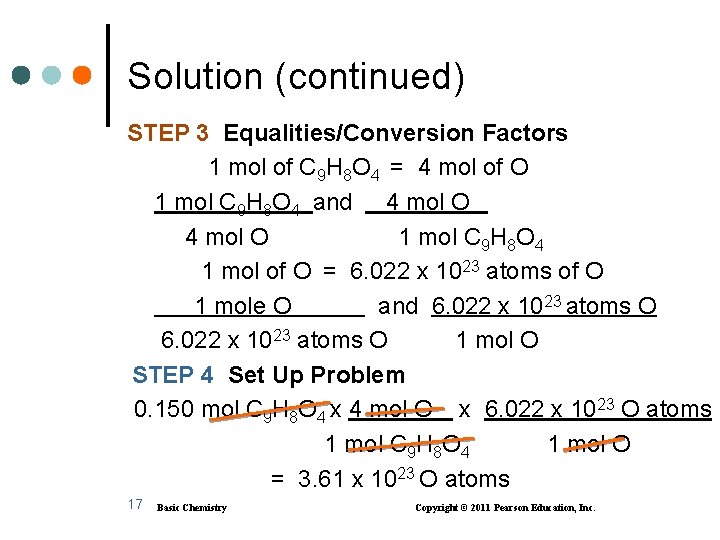
Solution (continued) STEP 3 Equalities/Conversion Factors 1 mol of C 9 H 8 O 4 = 4 mol of O 1 mol C 9 H 8 O 4 and 4 mol O 1 mol C 9 H 8 O 4 1 mol of O = 6. 022 x 1023 atoms of O 1 mole O and 6. 022 x 1023 atoms O 1 mol O STEP 4 Set Up Problem 0. 150 mol C 9 H 8 O 4 x 4 mol O x 6. 022 x 1023 O atoms 1 mol C 9 H 8 O 4 1 mol O = 3. 61 x 1023 O atoms 17 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Molar Mass Lithium carbonate produces a red color in fireworks. 18 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Molar Mass The molar mass • is the mass of one mol of an element or compound • is the atomic mass expressed in grams 19 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

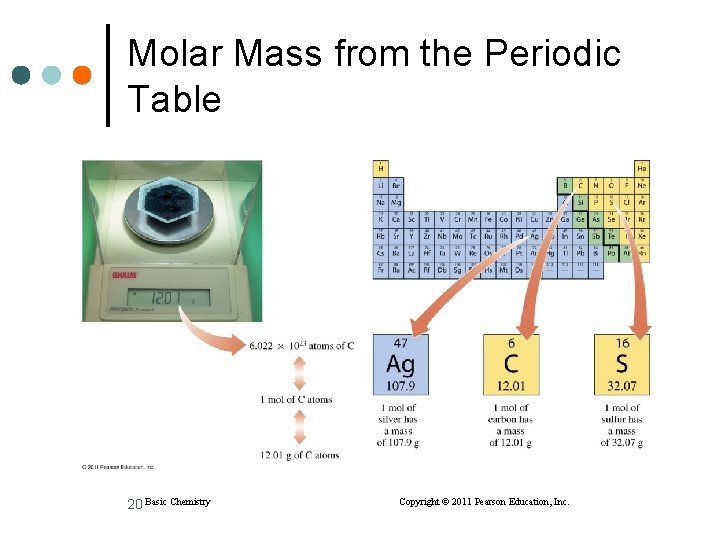
Molar Mass from the Periodic Table 20 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Learning Check Give the molar mass for A. 1 mol of K atoms = ____ B. 1 mol of Sn atoms = ____ 21 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Solution Give the molar mass for A. 1 mol of K atoms = 39. 10 g B. 1 mol of Sn atoms = 118. 7 g 22 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

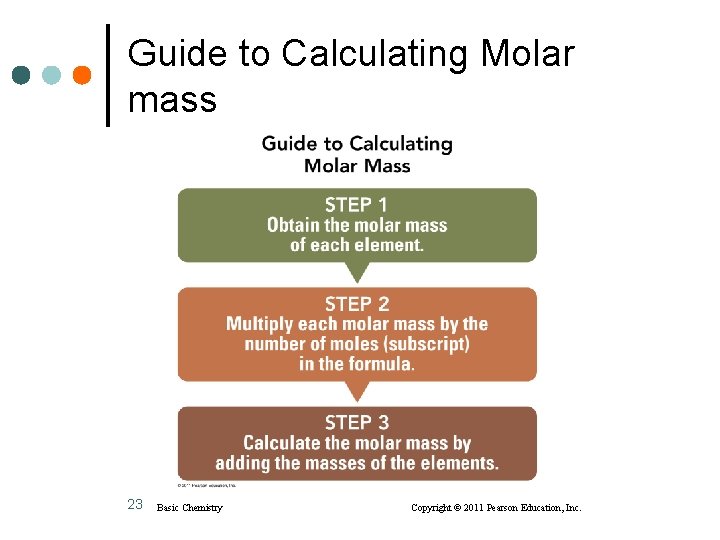
Guide to Calculating Molar mass 23 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

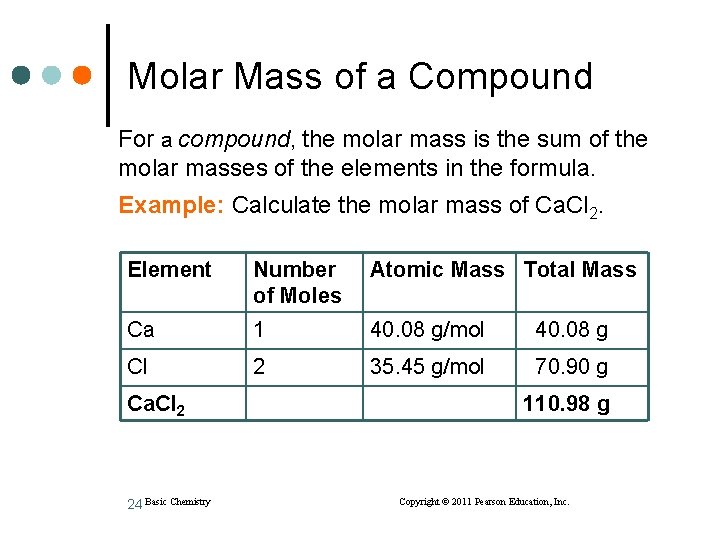
Molar Mass of a Compound For a compound, the molar mass is the sum of the molar masses of the elements in the formula. Example: Calculate the molar mass of Ca. Cl 2. Element Number of Moles Atomic Mass Total Mass Ca 1 40. 08 g/mol 40. 08 g Cl 2 35. 45 g/mol 70. 90 g Ca. Cl 2 24 Basic Chemistry 110. 98 g Copyright © 2011 Pearson Education, Inc.

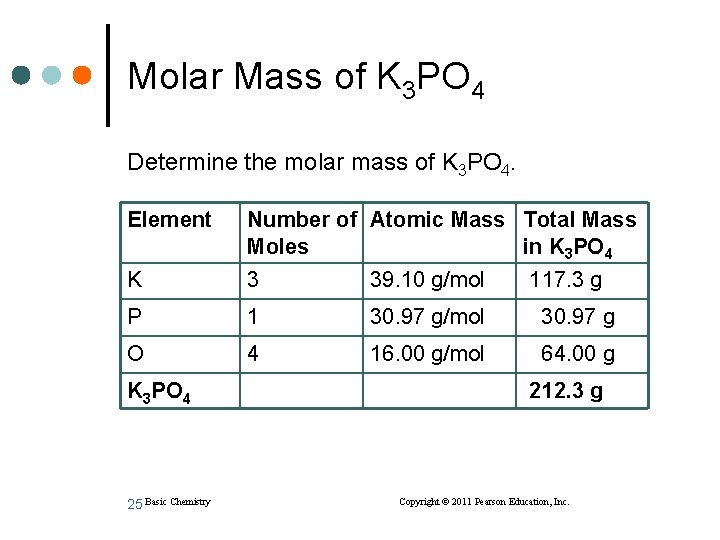
Molar Mass of K 3 PO 4 Determine the molar mass of K 3 PO 4. Element Number of Atomic Mass Total Mass Moles in K 3 PO 4 K 3 39. 10 g/mol P 1 30. 97 g/mol 30. 97 g O 4 16. 00 g/mol 64. 00 g K 3 PO 4 25 Basic Chemistry 117. 3 g 212. 3 g Copyright © 2011 Pearson Education, Inc.

Some One-Mol Quantities 26 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

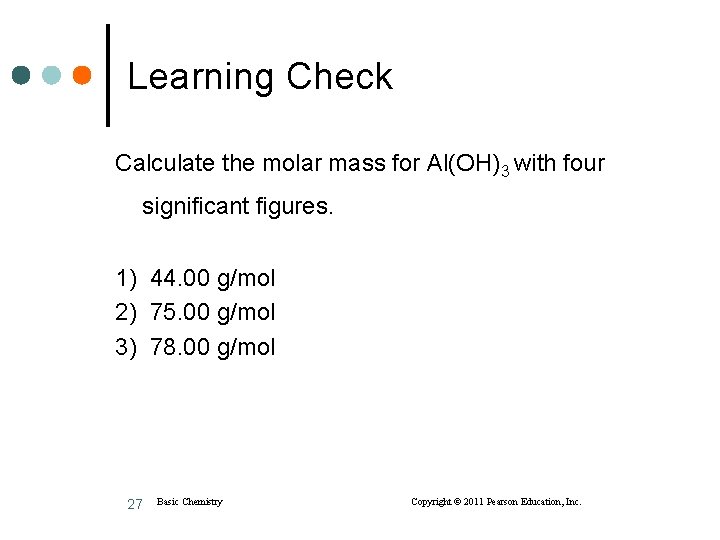
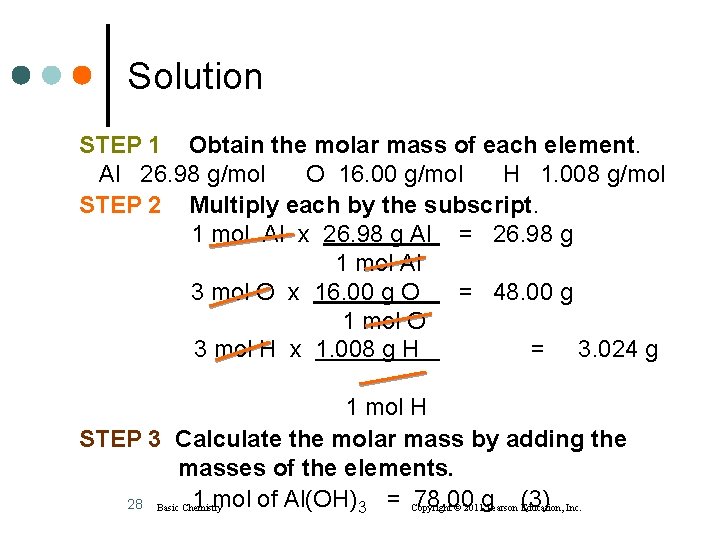
Learning Check Calculate the molar mass for Al(OH)3 with four significant figures. 1) 44. 00 g/mol 2) 75. 00 g/mol 3) 78. 00 g/mol 27 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Solution STEP 1 Obtain the molar mass of each element. Al 26. 98 g/mol O 16. 00 g/mol H 1. 008 g/mol STEP 2 Multiply each by the subscript. 1 mol Al x 26. 98 g Al = 26. 98 g 1 mol Al 3 mol O x 16. 00 g O = 48. 00 g 1 mol O 3 mol H x 1. 008 g H = 3. 024 g 1 mol H STEP 3 Calculate the molar mass by adding the masses of the elements. 1 mol of Al(OH)3 = Copyright 78. 00 28 Basic Chemistry © 2011 g Pearson (3) Education, Inc.

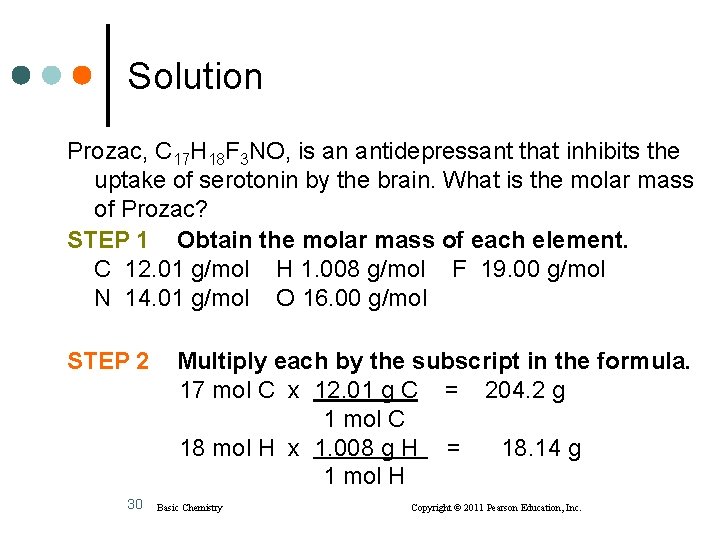
Learning Check Prozac, C 17 H 18 F 3 NO, is an antidepressant that inhibits the uptake of serotonin by the brain. What is the molar mass of Prozac? 1) 40. 06 g/mol 2) 262. 0 g/mol 3) 309. 4 g/mol 29 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

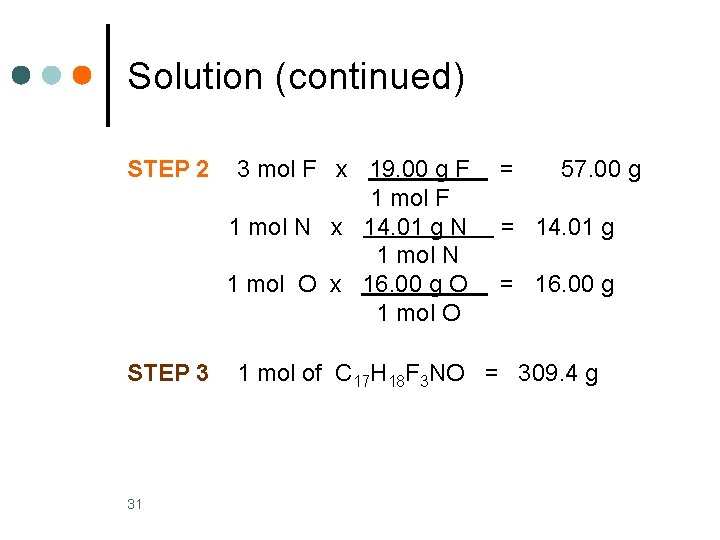
Solution Prozac, C 17 H 18 F 3 NO, is an antidepressant that inhibits the uptake of serotonin by the brain. What is the molar mass of Prozac? STEP 1 Obtain the molar mass of each element. C 12. 01 g/mol H 1. 008 g/mol F 19. 00 g/mol N 14. 01 g/mol O 16. 00 g/mol STEP 2 30 Multiply each by the subscript in the formula. 17 mol C x 12. 01 g C = 204. 2 g 1 mol C 18 mol H x 1. 008 g H = 18. 14 g 1 mol H Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Solution (continued) STEP 2 STEP 3 31 3 mol F x 19. 00 g F 1 mol N x 14. 01 g N 1 mol O x 16. 00 g O 1 mol O = 57. 00 g = 14. 01 g = 16. 00 g 1 mol of C 17 H 18 F 3 NO = 309. 4 g

Calculations Using Molar Mass Table salt is Na. Cl. 32 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

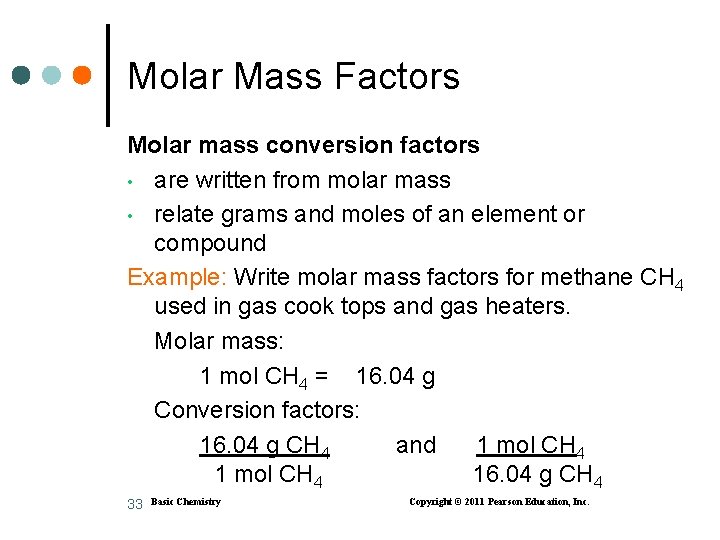
Molar Mass Factors Molar mass conversion factors • are written from molar mass • relate grams and moles of an element or compound Example: Write molar mass factors for methane CH 4 used in gas cook tops and gas heaters. Molar mass: 1 mol CH 4 = 16. 04 g Conversion factors: 16. 04 g CH 4 and 1 mol CH 4 16. 04 g CH 4 33 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Learning Check Acetic acid HC 2 H 3 O 2 gives the sour taste to vinegar. Write an equality and two molar mass conversion factors for acetic acid. 34 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Solution Acetic acid HC 2 H 3 O 2 gives the sour taste to vinegar. Write an equality and two molar mass conversion factors for acetic acid. Equality: 1 mol of acetic acid = 60. 05 g of acetic acid Molar mass conversion factors: 1 mol acetic acid and 60. 05 g acetic acid 1 mol acetic acid 35 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Guide to Calculation Using Molar Mass 36 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

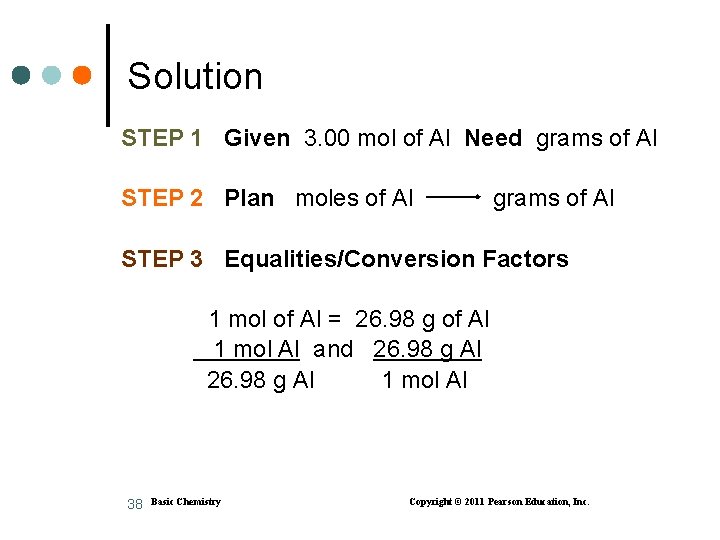
Moles to Grams Aluminum is used to build lightweight bicycle frames. How many grams of Al are in 3. 00 mol of Al? 37 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

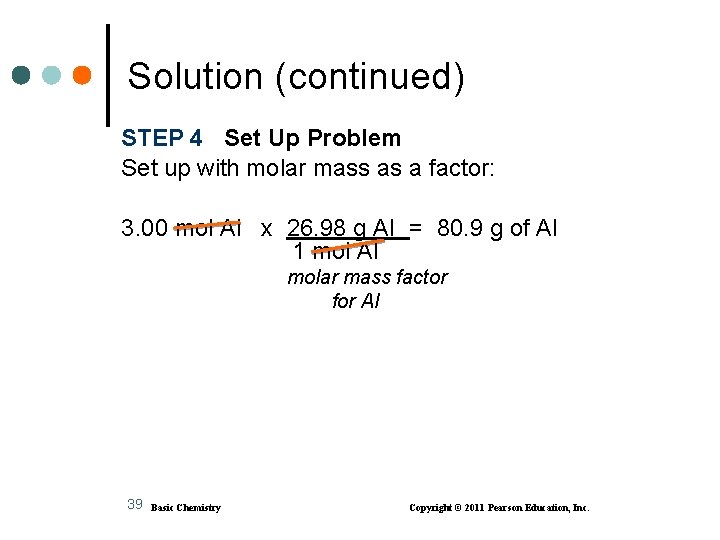
Solution STEP 1 Given 3. 00 mol of Al Need grams of Al STEP 2 Plan moles of Al grams of Al STEP 3 Equalities/Conversion Factors 1 mol of Al = 26. 98 g of Al 1 mol Al and 26. 98 g Al 1 mol Al 38 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Solution (continued) STEP 4 Set Up Problem Set up with molar mass as a factor: 3. 00 mol Al x 26. 98 g Al = 80. 9 g of Al 1 mol Al molar mass factor for Al 39 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Learning Check The artificial sweetener aspartame (Nutra. Sweet), C 14 H 18 N 2 O 5, is used to sweeten diet foods, coffee, and soft drinks. How many moles of aspartame are present in 225 g of aspartame? 40 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Solution STEP 1 Given 225 g of C 14 H 18 N 2 O 5 Need moles STEP 2 Plan g of aspartame moles of aspartame STEP 3 Equalities/Conversion Factors 1 mol aspartame = 294. 3 g 1 mol aspartame and 294. 3 g aspartame 1 mol aspartame STEP 4 Set Up Problem 225 g aspartame x 1 mol aspartame 294. 3 g aspartame mole factor (inverted) = 0. 765 mol of aspartame 41 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Learning Check Allyl sulfide, C 6 H 10 S, is a compound that has the odor of garlic. How many moles of C 6 H 10 S are in 225 g of C 6 H 10 S? 42 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

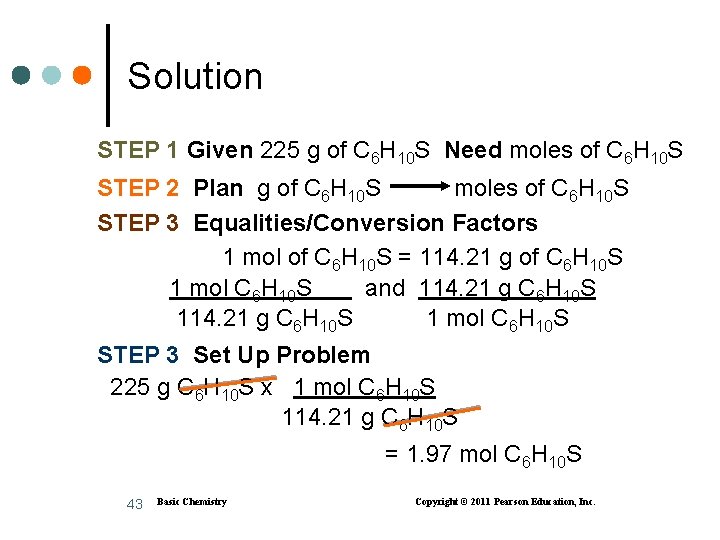
Solution STEP 1 Given 225 g of C 6 H 10 S Need moles of C 6 H 10 S STEP 2 Plan g of C 6 H 10 S moles of C 6 H 10 S STEP 3 Equalities/Conversion Factors 1 mol of C 6 H 10 S = 114. 21 g of C 6 H 10 S 1 mol C 6 H 10 S and 114. 21 g C 6 H 10 S 1 mol C 6 H 10 S STEP 3 Set Up Problem 225 g C 6 H 10 S x 1 mol C 6 H 10 S 114. 21 g C 6 H 10 S = 1. 97 mol C 6 H 10 S 43 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

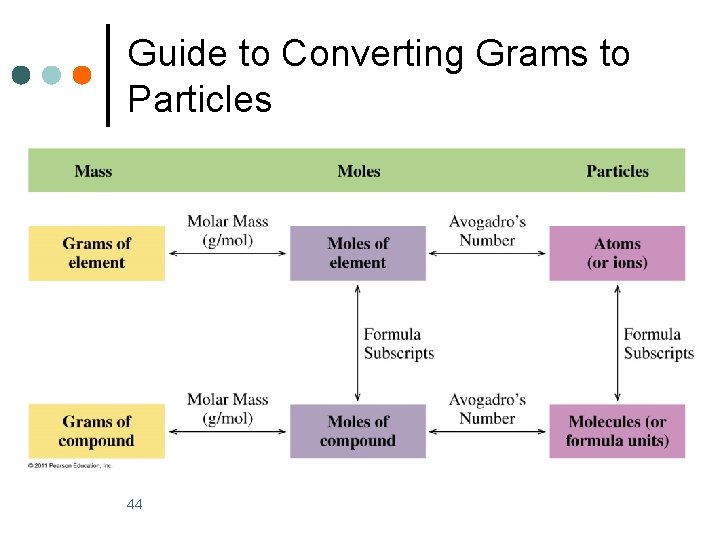
Guide to Converting Grams to Particles 44

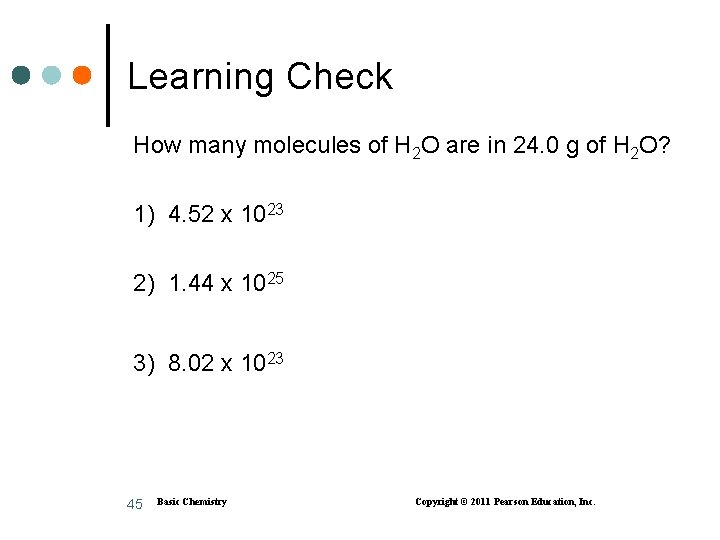
Learning Check How many molecules of H 2 O are in 24. 0 g of H 2 O? 1) 4. 52 x 1023 2) 1. 44 x 1025 3) 8. 02 x 1023 45 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

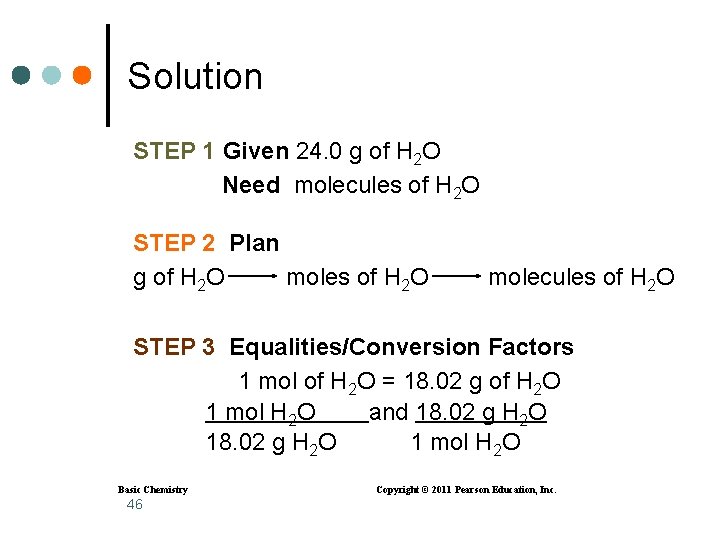
Solution STEP 1 Given 24. 0 g of H 2 O Need molecules of H 2 O STEP 2 Plan g of H 2 O moles of H 2 O molecules of H 2 O STEP 3 Equalities/Conversion Factors 1 mol of H 2 O = 18. 02 g of H 2 O 1 mol H 2 O and 18. 02 g H 2 O 1 mol H 2 O Basic Chemistry 46 Copyright © 2011 Pearson Education, Inc.

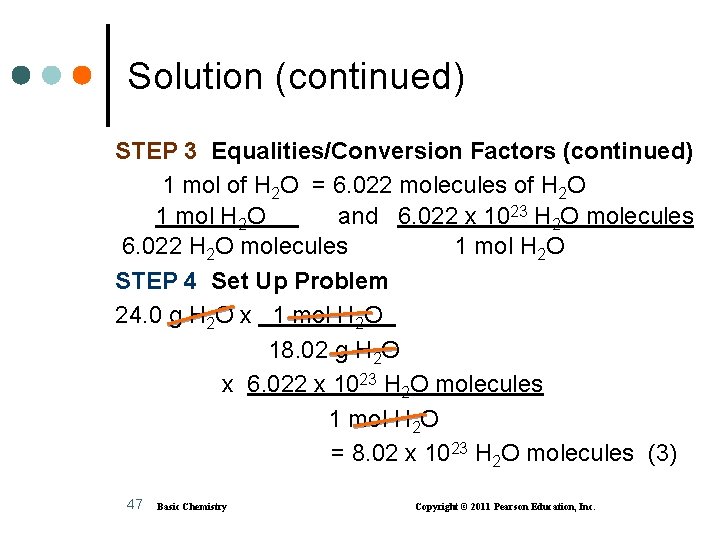
Solution (continued) STEP 3 Equalities/Conversion Factors (continued) 1 mol of H 2 O = 6. 022 molecules of H 2 O 1 mol H 2 O and 6. 022 x 1023 H 2 O molecules 6. 022 H 2 O molecules 1 mol H 2 O STEP 4 Set Up Problem 24. 0 g H 2 O x 1 mol H 2 O 18. 02 g H 2 O x 6. 022 x 1023 H 2 O molecules 1 mol H 2 O = 8. 02 x 1023 H 2 O molecules (3) 47 Basic Chemistry Copyright © 2011 Pearson Education, Inc.