Stoichiometry Definition n Reaction Stoichiometry is the calculation




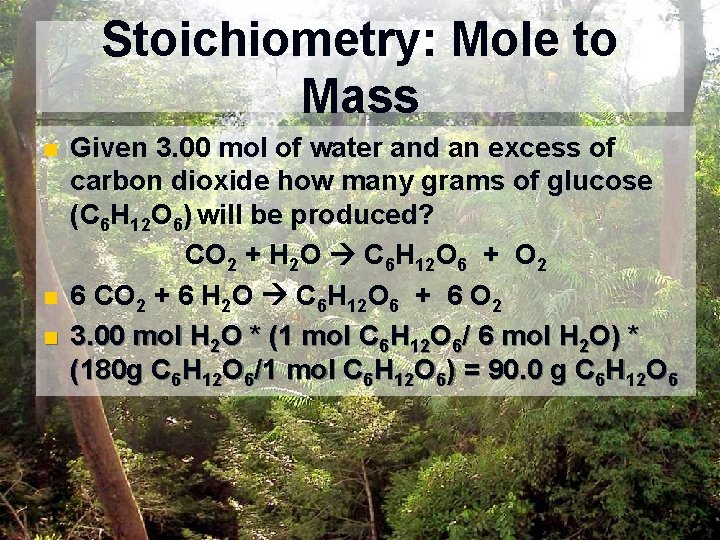








![Percent Yield n Percent Yield=[Actual Yield/Theoretical Yield] * Yield= 100% C 6 H 6 Percent Yield n Percent Yield=[Actual Yield/Theoretical Yield] * Yield= 100% C 6 H 6](https://slidetodoc.com/presentation_image/0038d6c75bc77f8a5a08605962adff35/image-21.jpg)
- Slides: 21

Stoichiometry

Definition n Reaction Stoichiometry is the calculation of quantitative (measurable) relationships of the reactants and products in chemical reactions

4 types of stoichiometry problems 1. 2. 3. 4. Mole – mole Mole – mass Mass – mole Mass - mass

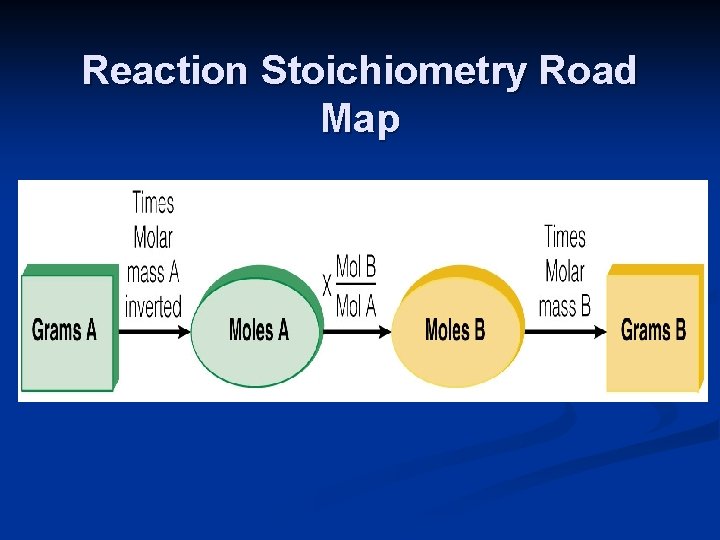
Reaction Stoichiometry Road Map

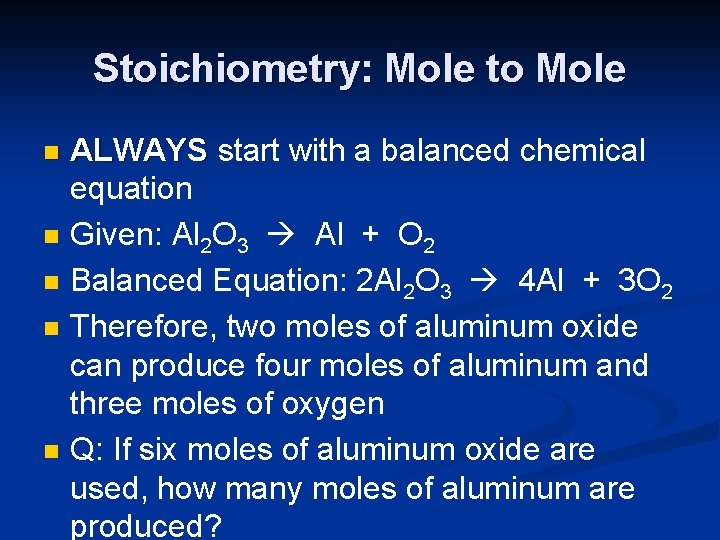
Stoichiometry: Mole to Mole n n n ALWAYS start with a balanced chemical equation Given: Al 2 O 3 Al + O 2 Balanced Equation: 2 Al 2 O 3 4 Al + 3 O 2 Therefore, two moles of aluminum oxide can produce four moles of aluminum and three moles of oxygen Q: If six moles of aluminum oxide are used, how many moles of aluminum are produced?

Problem Set-up n 2 Al 2 O 3 4 Al + 3 O 2 Start with the number in the question. n n Set-up a factor label problem, and move the units to the bottom to cancel n n 6 moles Al 2 O 3 X 4 moles Al 2 O 3 Complete the math, and add labels to your final answer. 24 = 12 moles of Al 2

YOU TRY Balance your equation left to right: CO 2 + Li. OH Li 2 CO 3 + H 2 O n CO 2 + 2 Li. OH Li 2 CO 3 + H 2 O n How many moles of Li. OH are required to react with 30 mol of CO 2? n 60 moles of Li. OH n
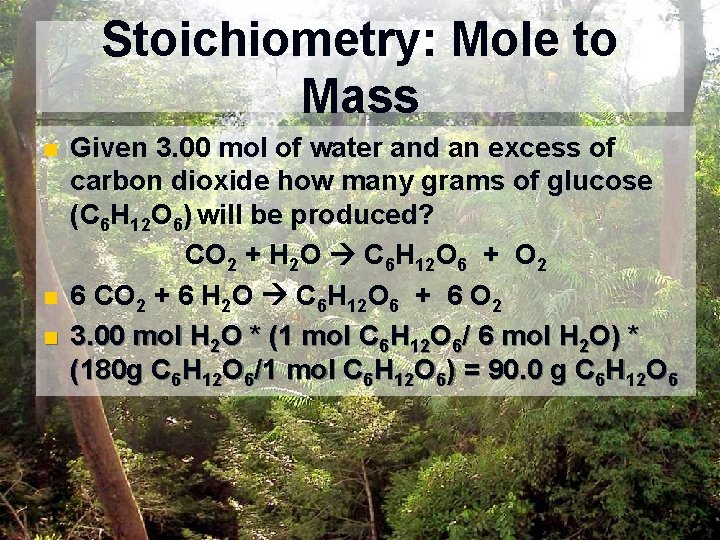
Stoichiometry: Mole to Mass n n n Given 3. 00 mol of water and an excess of carbon dioxide how many grams of glucose (C 6 H 12 O 6) will be produced? CO 2 + H 2 O C 6 H 12 O 6 + O 2 6 CO 2 + 6 H 2 O C 6 H 12 O 6 + 6 O 2 3. 00 mol H 2 O * (1 mol C 6 H 12 O 6/ 6 mol H 2 O) * (180 g C 6 H 12 O 6/1 mol C 6 H 12 O 6) = 90. 0 g C 6 H 12 O 6

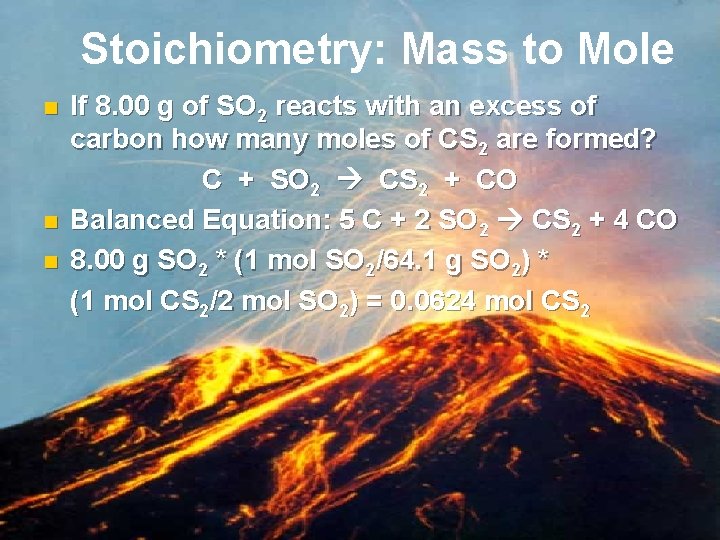
Stoichiometry: Mass to Mole n n n If 8. 00 g of SO 2 reacts with an excess of carbon how many moles of CS 2 are formed? C + SO 2 CS 2 + CO Balanced Equation: 5 C + 2 SO 2 CS 2 + 4 CO 8. 00 g SO 2 * (1 mol SO 2/64. 1 g SO 2) * (1 mol CS 2/2 mol SO 2) = 0. 0624 mol CS 2

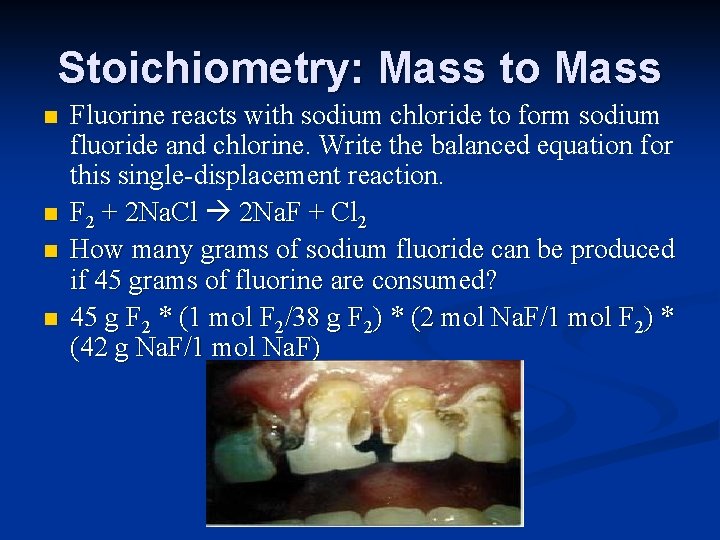
Stoichiometry: Mass to Mass n n Fluorine reacts with sodium chloride to form sodium fluoride and chlorine. Write the balanced equation for this single-displacement reaction. F 2 + 2 Na. Cl 2 Na. F + Cl 2 How many grams of sodium fluoride can be produced if 45 grams of fluorine are consumed? 45 g F 2 * (1 mol F 2/38 g F 2) * (2 mol Na. F/1 mol F 2) * (42 g Na. F/1 mol Na. F)

Limiting reactant n Reactant that limits the amount of the other reactants used and the amount of product formed

Limiting Reactant

Everyday Example n Nestle Toll House® Chocolate Chip Cookie Recipe n n n n n 2 1/4 cups all-purpose flour 1 teaspoon baking soda 1 teaspoon salt 1 cup (2 sticks, 1/2 pound) butter, softened 3/4 cup granulated [white] sugar 3/4 cup packed brown sugar 1 teaspoon vanilla extract 2 eggs 2 cups (12 -ounce package) NESTLE TOLL HOUSE Semi. Sweet Chocolate Morsels 1 cup chopped nuts n n n If you have 40 ounces of semisweet chocolate morsels, 6 cups of chopped nuts, 4. 5 teaspoons of salt, and 1. 5 cups of brown sugar how many batches of cookies can you bake with each ingredient? 40 ounces of morsels = 3 batches 6 cups of nuts = 6 batches 4. 5 teaspoons of salt = 4 batches 1. 5 cups sugar = 2 batches You can only make two batches of cookies. After two batches you will run short on needed ingredients.

Everyday Example #2 The Hollidaysburg Area chemistry club decide to prepare fruit baskets as a fund raiser. A complete fruit basket contains six oranges, two bananas, one pineapple, and three grapefruit. n The secretary of the club placed the following order with out consulting their chief of stoichiometric affairs: 5 dozen oranges, 2 dozen bananas, 1 dozen pineapples, and 2 dozen grapefruits. n

Everyday Example #2 Needed: 6 oranges, 2 bananas, 1 pineapple, and 3 grapefruit n Ordered: 5 dozen oranges, 2 dozen bananas, 1 dozen pineapples, and 2 dozen grapefruits n Write a balanced equation showing the correct ratios relating the reactants and product in the preparation of a fruit basket. n 6 O + 2 B + 1 P + 3 G 1 Basket n

Everyday Example #2 n How many complete fruit baskets can be prepared? n n n Example Calculation: n n n Ordered: 5 dozen oranges, 2 dozen bananas, 1 dozen pineapples, and 2 dozen grapefruits 6 O + 2 B + 1 P + 3 G 1 Basket 5 dozen oranges = 60 oranges x 1 basket = 10 baskets 6 oranges Bananas = 12 baskets Pineapples = 12 baskets Grapefruit = 8 baskets

Everyday Example #2 n n What fruit is the limiting reagent? GRAPEFRUIT How much of the other fruits are left over? Example Calculation n n 8 baskets x 6 oranges = 48 oranges 1 basket 60 oranges ordered – 48 oranges used = 12 oranges left 8 bananas 4 pineapples

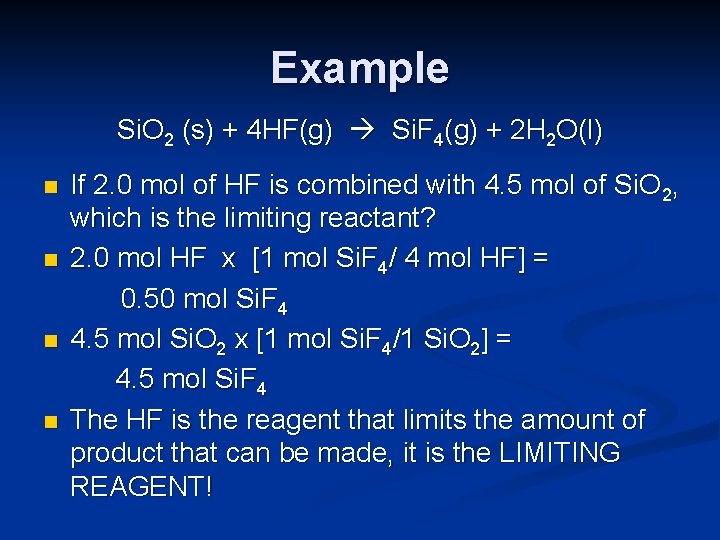
Chemical Example n Silicon dioxide (quartz) reacts with hydrogen fluoride according to the following reaction: Si. O 2 (s) + 4 HF(g) Si. F 4(g) + 2 H 2 O(l)

Example Si. O 2 (s) + 4 HF(g) Si. F 4(g) + 2 H 2 O(l) n n If 2. 0 mol of HF is combined with 4. 5 mol of Si. O 2, which is the limiting reactant? 2. 0 mol HF x [1 mol Si. F 4/ 4 mol HF] = 0. 50 mol Si. F 4 4. 5 mol Si. O 2 x [1 mol Si. F 4/1 Si. O 2] = 4. 5 mol Si. F 4 The HF is the reagent that limits the amount of product that can be made, it is the LIMITING REAGENT!

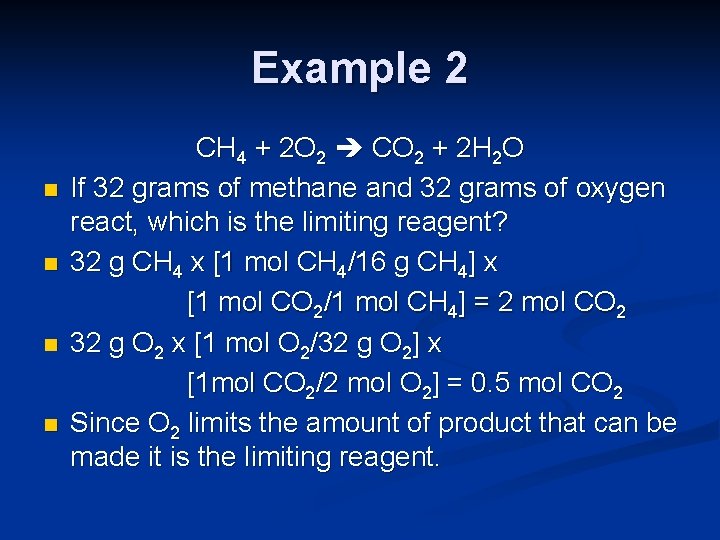
Example 2 n n CH 4 + 2 O 2 CO 2 + 2 H 2 O If 32 grams of methane and 32 grams of oxygen react, which is the limiting reagent? 32 g CH 4 x [1 mol CH 4/16 g CH 4] x [1 mol CO 2/1 mol CH 4] = 2 mol CO 2 32 g O 2 x [1 mol O 2/32 g O 2] x [1 mol CO 2/2 mol O 2] = 0. 5 mol CO 2 Since O 2 limits the amount of product that can be made it is the limiting reagent.
![Percent Yield n Percent YieldActual YieldTheoretical Yield Yield 100 C 6 H 6 Percent Yield n Percent Yield=[Actual Yield/Theoretical Yield] * Yield= 100% C 6 H 6](https://slidetodoc.com/presentation_image/0038d6c75bc77f8a5a08605962adff35/image-21.jpg)
Percent Yield n Percent Yield=[Actual Yield/Theoretical Yield] * Yield= 100% C 6 H 6 + Cl 2 C 6 H 5 Cl + HCl n If 36. 8 g of C 6 H 6 reacts with an excess of Cl 2 the actual yield is 38. 8 g of C 6 H 5 Cl. What is the percent yield of this reaction? n 36. 8 g C 6 H 6 * [1 mol C 6 H 6/78. 1 g C 6 H 6] * [1 mol C 6 H 5 Cl/1 mol C 6 H 6] * [113 g C 6 H 5 Cl/1 mol C 6 H 5 Cl] = 53. 2 g C 6 H 5 Cl n