Solution Stoichiometry Workshop 1 Molarity is a term







- Slides: 21

Solution Stoichiometry Workshop 1

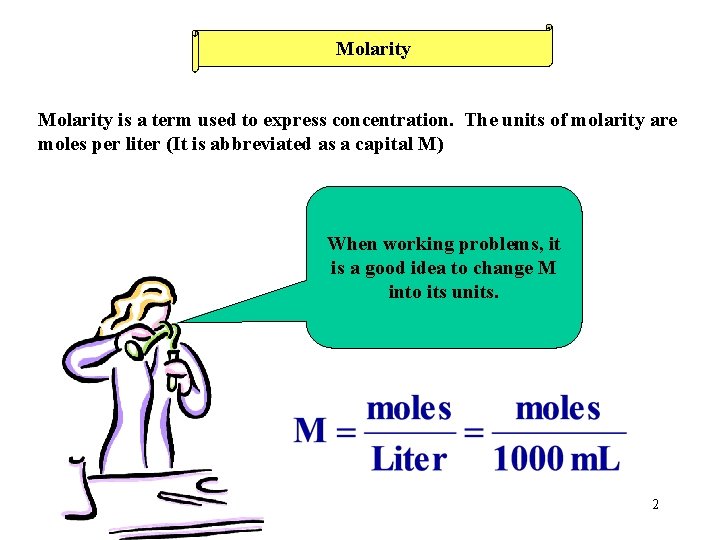
Molarity is a term used to express concentration. The units of molarity are moles per liter (It is abbreviated as a capital M) When working problems, it is a good idea to change M into its units. 2

Solutions A solution is prepared by dissolving 3. 73 grams of Al. Cl 3 in water to form 200. 0 m. L solution. A 10. 0 m. L portion of the solution is then used to prepare 100. 0 m. L of solution. Determine the molarity of the final solution. What type of problem(s) is this? Molarity followed by dilution. 3

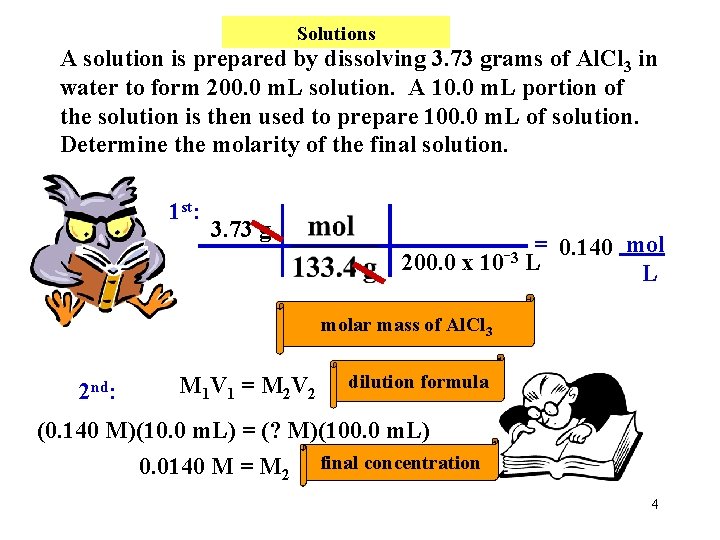
Solutions A solution is prepared by dissolving 3. 73 grams of Al. Cl 3 in water to form 200. 0 m. L solution. A 10. 0 m. L portion of the solution is then used to prepare 100. 0 m. L of solution. Determine the molarity of the final solution. 1 st: 3. 73 g = 0. 140 mol 3 200. 0 x 10 L L molar mass of Al. Cl 3 2 nd: M 1 V 1 = M 2 V 2 dilution formula (0. 140 M)(10. 0 m. L) = (? M)(100. 0 m. L) 0. 0140 M = M 2 final concentration 4

5

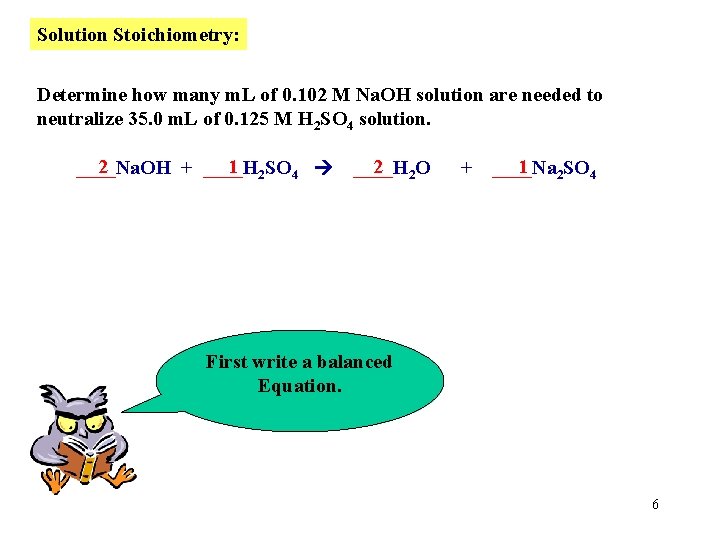
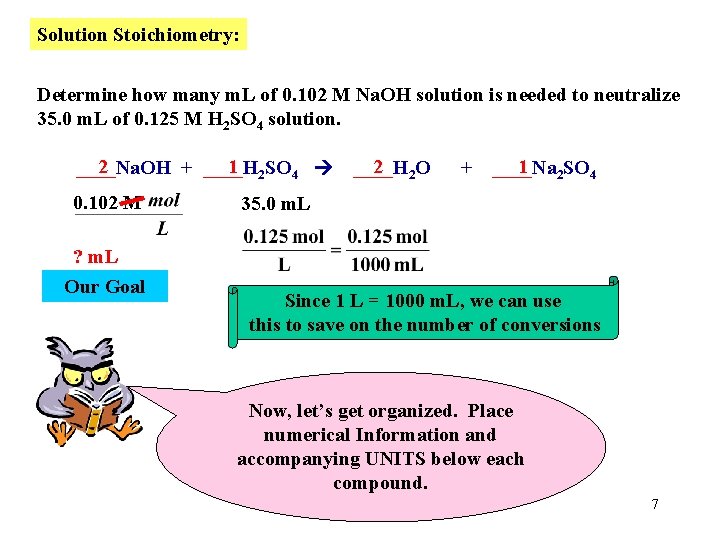
Solution Stoichiometry: Determine how many m. L of 0. 102 M Na. OH solution are needed to neutralize 35. 0 m. L of 0. 125 M H 2 SO 4 solution. 2 1 2 SO 4 ____Na. OH + ____H 2 2 O ____H + 1 2 SO 4 ____Na First write a balanced Equation. 6

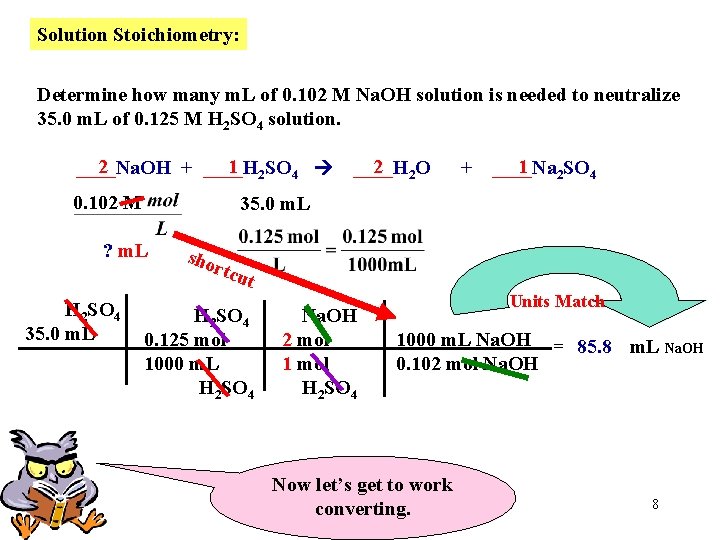
Solution Stoichiometry: Determine how many m. L of 0. 102 M Na. OH solution is needed to neutralize 35. 0 m. L of 0. 125 M H 2 SO 4 solution. 2 1 2 SO 4 ____Na. OH + ____H 0. 102 M 2 2 O ____H + 1 2 SO 4 ____Na 35. 0 m. L ? m. L Our Goal Since 1 L = 1000 m. L, we can use this to save on the number of conversions Now, let’s get organized. Place numerical Information and accompanying UNITS below each compound. 7

Solution Stoichiometry: Determine how many m. L of 0. 102 M Na. OH solution is needed to neutralize 35. 0 m. L of 0. 125 M H 2 SO 4 solution. 2 1 2 SO 4 ____Na. OH + ____H 0. 102 M + 1 2 SO 4 ____Na 35. 0 m. L ? m. L H 2 SO 4 35. 0 m. L 2 2 O ____H sho rtcu t H 2 SO 4 0. 125 mol 1000 m. L H 2 SO 4 Na. OH 2 mol 1 mol H 2 SO 4 Units Match 1000 m. L Na. OH = 85. 8 m. L Na. OH 0. 102 mol Na. OH Now let’s get to work converting. 8

9

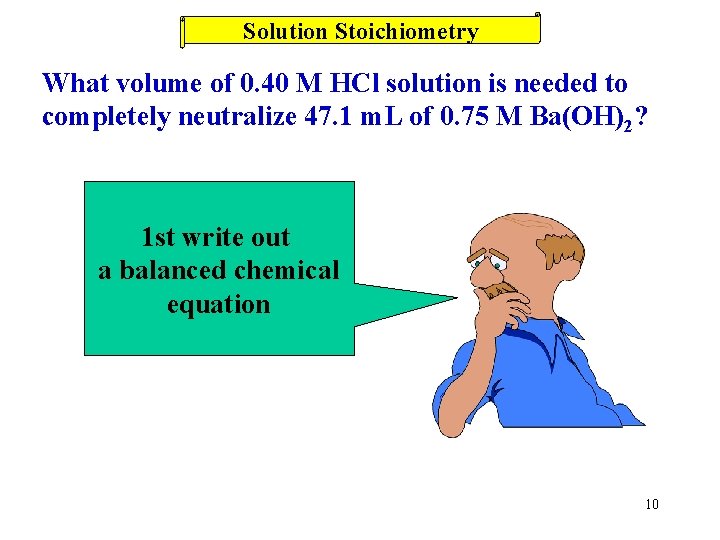
Solution Stoichiometry What volume of 0. 40 M HCl solution is needed to completely neutralize 47. 1 m. L of 0. 75 M Ba(OH)2? 1 st write out a balanced chemical equation 10

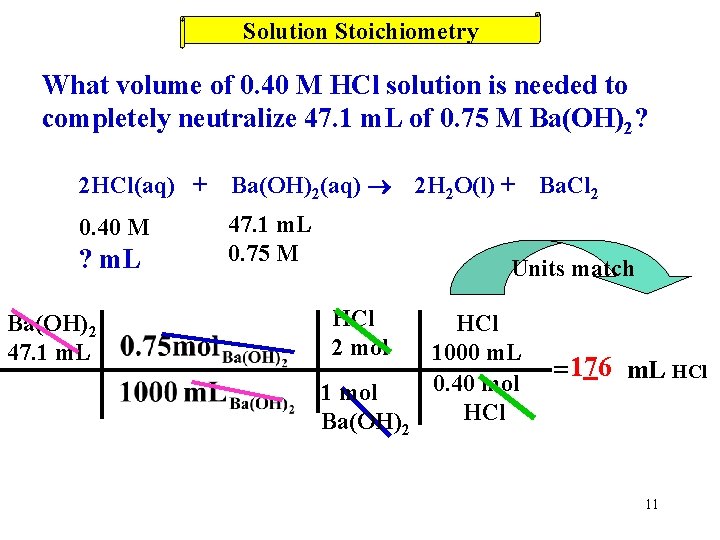
Solution Stoichiometry What volume of 0. 40 M HCl solution is needed to completely neutralize 47. 1 m. L of 0. 75 M Ba(OH)2? 2 HCl(aq) + Ba(OH)2(aq) 0. 40 M 47. 1 m. L 0. 75 M ? m. L Ba(OH)2 47. 1 m. L 2 H 2 O(l) + Ba. Cl 2 Units match HCl 2 mol 1 mol Ba(OH)2 HCl 1000 m. L 0. 40 mol HCl = 176 m. L HCl 11

12

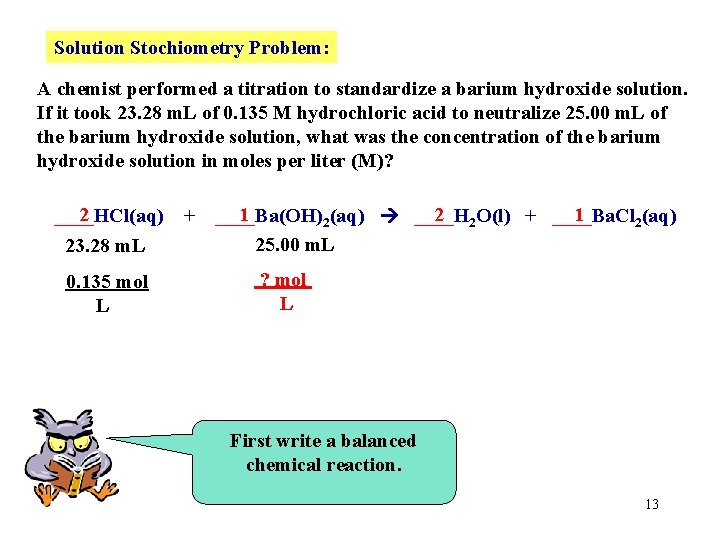
Solution Stochiometry Problem: A chemist performed a titration to standardize a barium hydroxide solution. If it took 23. 28 m. L of 0. 135 M hydrochloric acid to neutralize 25. 00 m. L of the barium hydroxide solution, what was the concentration of the barium hydroxide solution in moles per liter (M)? 2 ____HCl(aq) 23. 28 m. L 0. 135 mol L + 1 2 2 O(l) + ____Ba. Cl 1 ____Ba(OH) 2(aq) ____H 2(aq) 25. 00 m. L ? mol L First write a balanced chemical reaction. 13

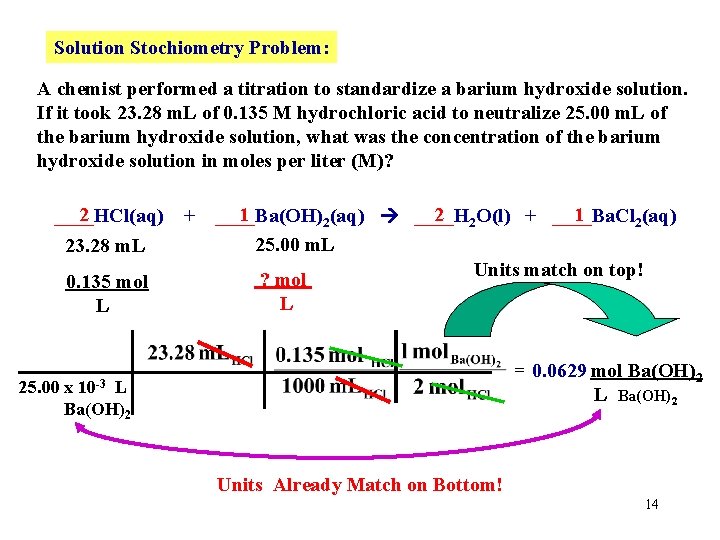
Solution Stochiometry Problem: A chemist performed a titration to standardize a barium hydroxide solution. If it took 23. 28 m. L of 0. 135 M hydrochloric acid to neutralize 25. 00 m. L of the barium hydroxide solution, what was the concentration of the barium hydroxide solution in moles per liter (M)? 2 ____HCl(aq) 23. 28 m. L 0. 135 mol L + 1 2 2 O(l) + ____Ba. Cl 1 ____Ba(OH) 2(aq) ____H 2(aq) 25. 00 m. L Units match on top! ? mol L = 0. 0629 mol Ba(OH)2 L Ba(OH)2 10 -3 25. 00 x L Ba(OH)2 Units Already Match on Bottom! 14

15

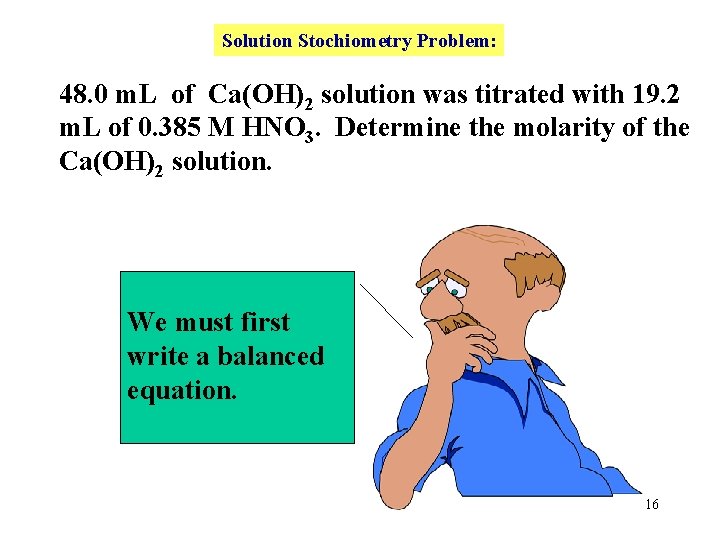
Solution Stochiometry Problem: 48. 0 m. L of Ca(OH)2 solution was titrated with 19. 2 m. L of 0. 385 M HNO 3. Determine the molarity of the Ca(OH)2 solution. We must first write a balanced equation. 16

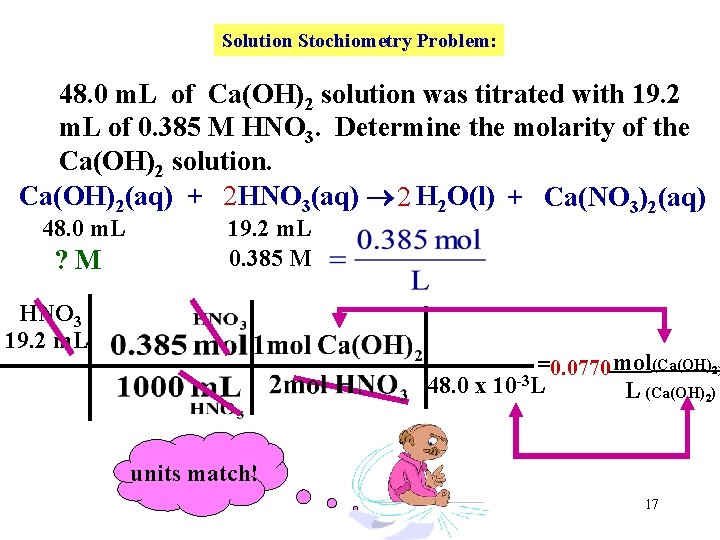
Solution Stochiometry Problem: 48. 0 m. L of Ca(OH)2 solution was titrated with 19. 2 m. L of 0. 385 M HNO 3. Determine the molarity of the Ca(OH)2 solution. Ca(OH)2(aq) + 2 HNO 3(aq) 2 H 2 O(l) + Ca(NO 3)2(aq) 48. 0 m. L ? M 19. 2 m. L 0. 385 M HNO 3 19. 2 m. L =0. 0770 mol(Ca(OH)2) 48. 0 x 10 -3 L L (Ca(OH)2) units match! 17

18

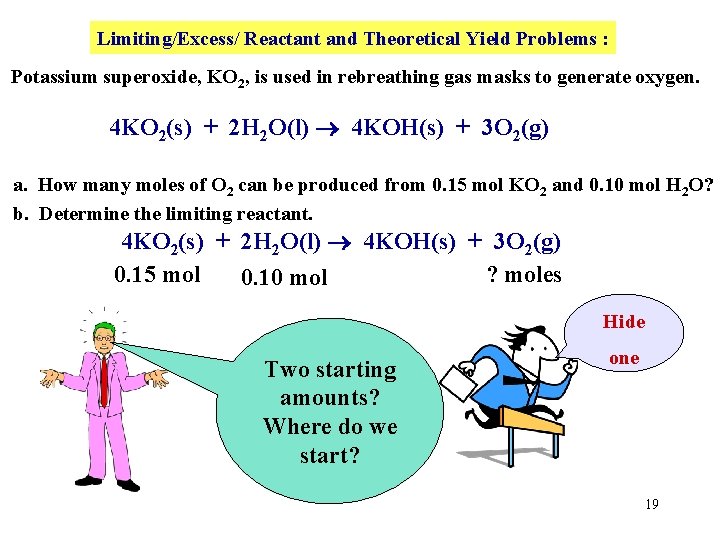
Limiting/Excess/ Reactant and Theoretical Yield Problems : Potassium superoxide, KO 2, is used in rebreathing gas masks to generate oxygen. 4 KO 2(s) + 2 H 2 O(l) 4 KOH(s) + 3 O 2(g) a. How many moles of O 2 can be produced from 0. 15 mol KO 2 and 0. 10 mol H 2 O? b. Determine the limiting reactant. 4 KO 2(s) + 2 H 2 O(l) 4 KOH(s) + 3 O 2(g) 0. 15 mol ? moles 0. 10 mol Hide Two starting amounts? Where do we start? one 19

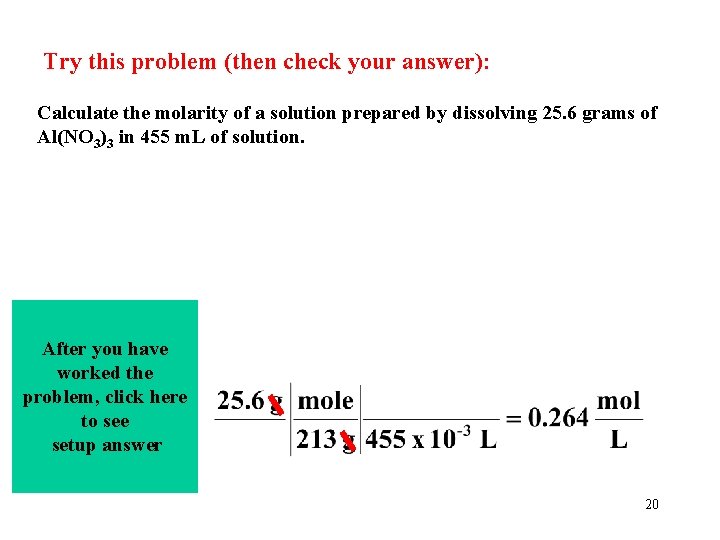
Try this problem (then check your answer): Calculate the molarity of a solution prepared by dissolving 25. 6 grams of Al(NO 3)3 in 455 m. L of solution. After you have worked the problem, click here to see setup answer 20

21