Stoichiometry Ch 12 Page 352 What is stoichiometry


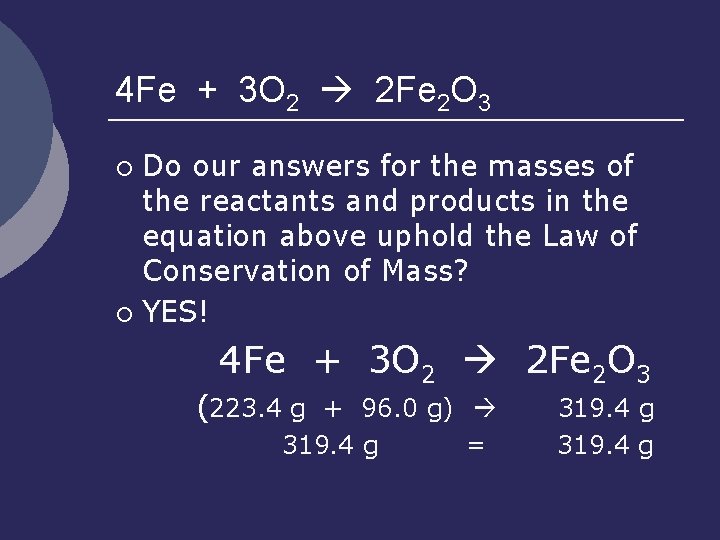











- Slides: 22

Stoichiometry Ch. 12 Page 352

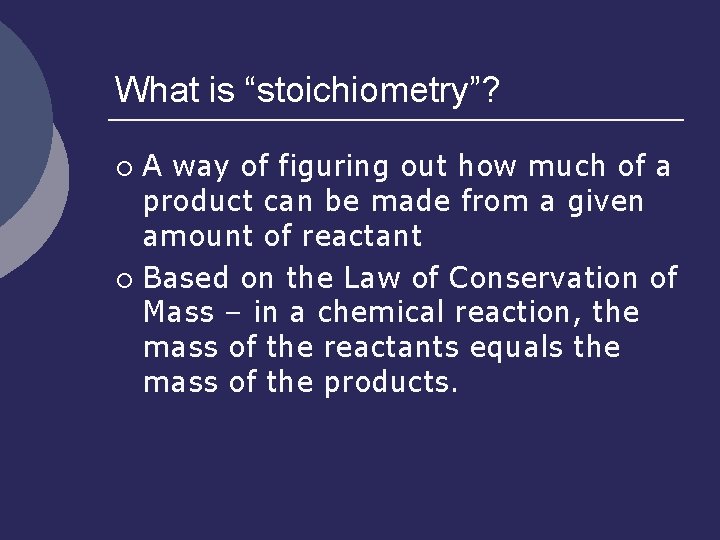
What is “stoichiometry”? A way of figuring out how much of a product can be made from a given amount of reactant ¡ Based on the Law of Conservation of Mass – in a chemical reaction, the mass of the reactants equals the mass of the products. ¡

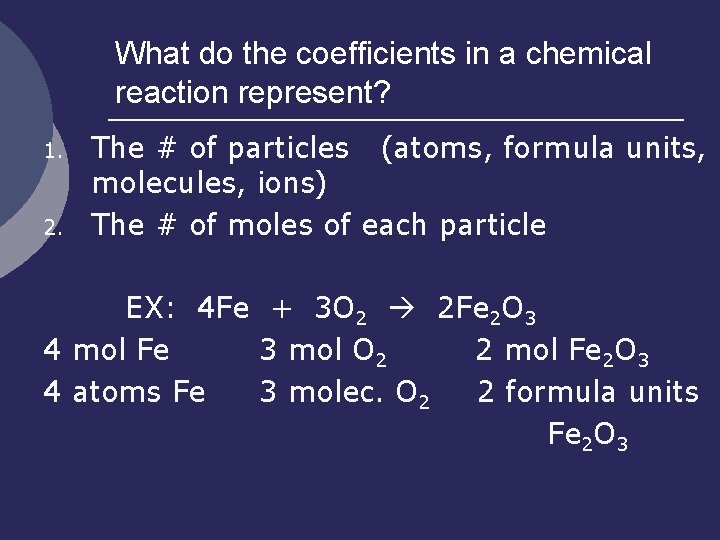
What do the coefficients in a chemical reaction represent? 1. 2. The # of particles (atoms, formula units, molecules, ions) The # of moles of each particle EX: 4 Fe + 3 O 2 2 Fe 2 O 3 4 mol Fe 3 mol O 2 2 mol Fe 2 O 3 4 atoms Fe 3 molec. O 2 2 formula units Fe 2 O 3

4 Fe + 3 O 2 2 Fe 2 O 3 A balanced chemical equation ALSO tells us indirectly about the masses of the reactants and products (using the # moles of each substance and the molar masses). ¡ How many grams of Fe are used in this reaction? ¡ How many grams of O 2? ¡ How many grams of Fe 2 O 3? (Let’s do these on the board. ) ¡
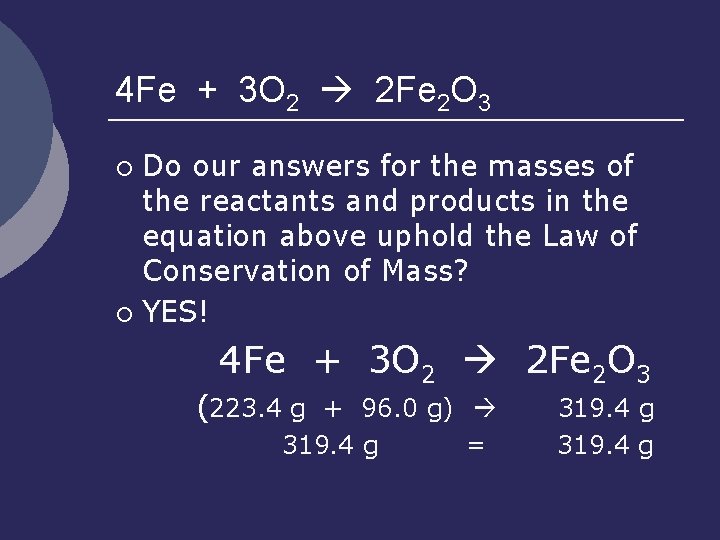
4 Fe + 3 O 2 2 Fe 2 O 3 Do our answers for the masses of the reactants and products in the equation above uphold the Law of Conservation of Mass? ¡ YES! ¡ 4 Fe + 3 O 2 2 Fe 2 O 3 (223. 4 g + 96. 0 g) 319. 4 g = 319. 4 g

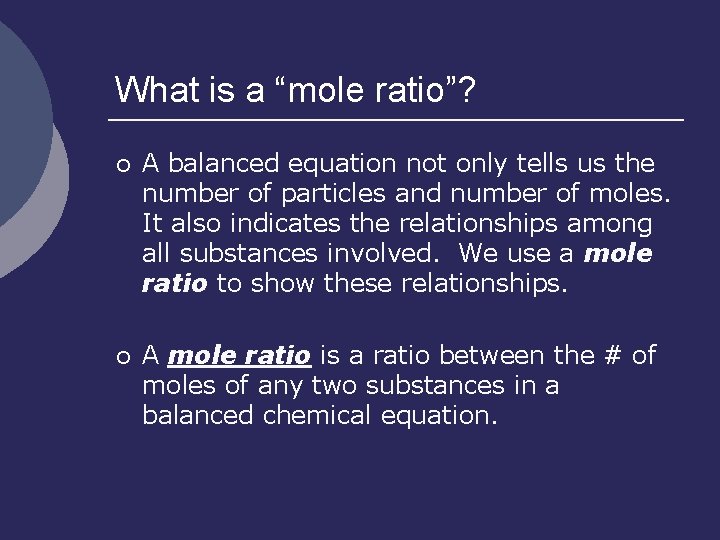
What is a “mole ratio”? ¡ A balanced equation not only tells us the number of particles and number of moles. It also indicates the relationships among all substances involved. We use a mole ratio to show these relationships. ¡ A mole ratio is a ratio between the # of moles of any two substances in a balanced chemical equation.

Mole Ratio: the “Bridge” MOLE RATIOS ARE THE KEY TO CALCULATIONS BASED UPON A CHEMICAL EQUATION! ¡ From a balanced chemical equation, if you know the amount of one reactant, you can calculate the amount of any other reactant in the equation and the maximum amount of product you can obtain. ¡ The “mole ratios” are the bridges we use to get from one kind of substance to another. ¡

4 Fe + 3 O 2 2 Fe 2 O 3 How do you know how many mole ratios are available in an equation? ? ? Just multiply the total number of substances (n) by the next lower number (n-1). ¡ From this equation, there are 3 substances. Multiply 3 x 2 and you will get 6 available mole ratios. ¡ Using the equation above, let’s write all the available mole ratios (on the board). ¡

Sec. 2: Using Stoichiometric Calculations ¡ 1. 2. 3. What are the tools needed for stoichiometric calculations? A balanced chemical equation Mole ratios Mass-to-mole conversions

Types of Stoichiometric Calculations ¡ 1. 2. 3. There are 3 types of stoichiometric calculations we will discuss: mole-to-mole conversions mole-to-mass conversions mass-to-mass conversions For all of these conversions, we will use the following flow-chart:

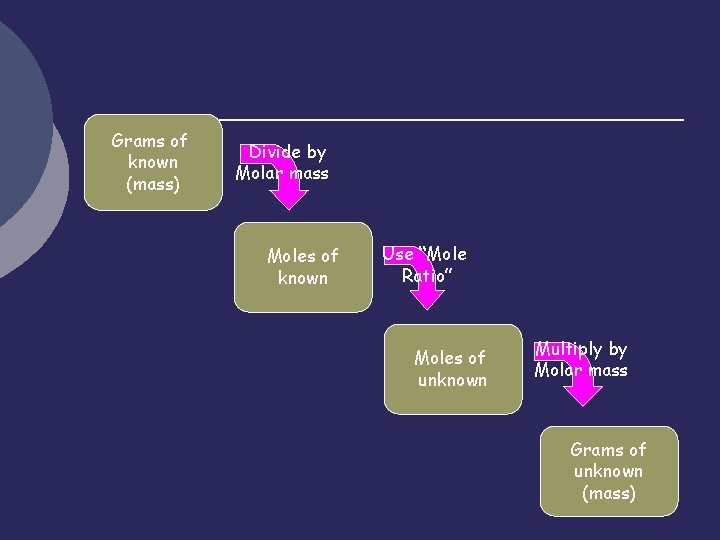
Grams of known (mass) Divide by Molar mass Moles of known Use “Mole Ratio” Moles of unknown Multiply by Molar mass Grams of unknown (mass)

Steps in Stoichiometric Calculations 1. 2. 3. 4. Write a balanced chemical equation. Determine the moles of the known using the mass-mole conversion Determine the moles of the unknown substance by using a mole ratio Determine the mass of the unknown using a mole-mass conversion

Complete the following Practice Problems Page 877: #5 – 10 ¡ Page 379: #67 – 68 ¡ Page 380: #72 -73 ¡

12. 3: Limiting Reactants, pg. 364 Rarely in nature are reactants in a chemical reaction present in the exact ratios specified by the balanced equation. ¡ Generally, one or more reactants are in excess and the reaction proceeds until all of one reactant is used up. ¡

When a chemical reaction is carried out in the laboratory, the same principle applies. ¡ Usually, one or more reactants are in excess, while one is limited. ¡ The amount of product depends upon the reactant that is limited. ¡

The limiting reactant limits the extent of the reaction and, thereby, determines the amount of product. ¡ A portion of the other reactants remains after the reaction stops. ¡ These left-over reactants are called excess reactants. ¡

In section 2 of this chapter, we did calculations based on having the reactants present in the ratio described by the balanced chemical equation. ¡ How can you calculate the amount of product formed when one reactant limits the amount of product and the other is in excess? ? ¡

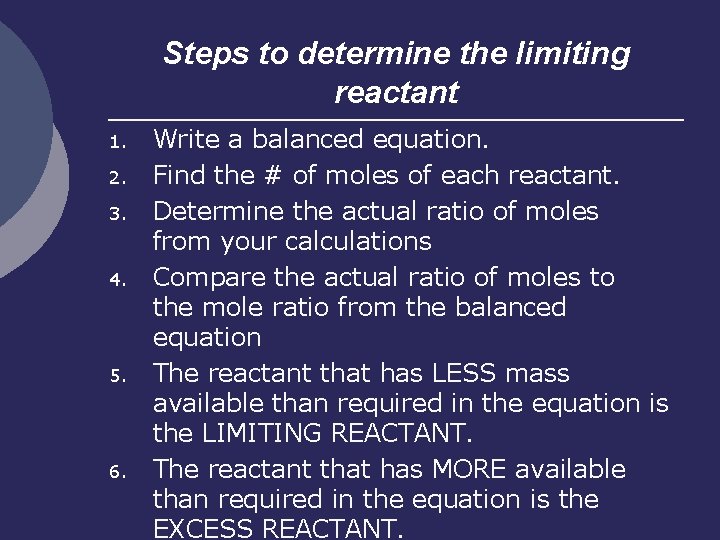
Steps to determine the limiting reactant 1. 2. 3. 4. 5. 6. Write a balanced equation. Find the # of moles of each reactant. Determine the actual ratio of moles from your calculations Compare the actual ratio of moles to the mole ratio from the balanced equation The reactant that has LESS mass available than required in the equation is the LIMITING REACTANT. The reactant that has MORE available than required in the equation is the EXCESS REACTANT.

To determine the mass of the excess reactant After determining the limiting reactant, do a mole-mass calculation (moles of limiting reactant grams excess reactant) (THIS IS HOW MUCH OF THE EXCESS REACTANT ACTUALLY REACTS. ) 2. Subtract that answer from the amount available for the reaction and you will have the mass of excess reactant left over. 1.

Steps to determine the amount of product from the reaction 1. 2. Determine the limiting reactant Do a mole-mass calculation (moles of limiting reactant grams product)

12. 4: Percent Yield In many calculations we have been practicing, we have been asked to calculate the amount of product that can be produced from a given amount of reactant. ¡ The answer we obtained is called theoretical yield: the max. amount of product that can be produced from a given amount of reactant. ¡

A chemical reaction rarely produces theoretical yield of product. ¡ When we conduct experiments, we determine the actual yield: the amount of product actually produced when the chemical reaction is carefully carried out in an experiment. ¡ We measure efficiency by calculating percent yield with the following formula: ¡ % yield = __actual yield (from experiment)_ x 100 theoretical yield (from calculations)