Chapter 11 Stoichiometry Sec 11 3 Limiting Reactants







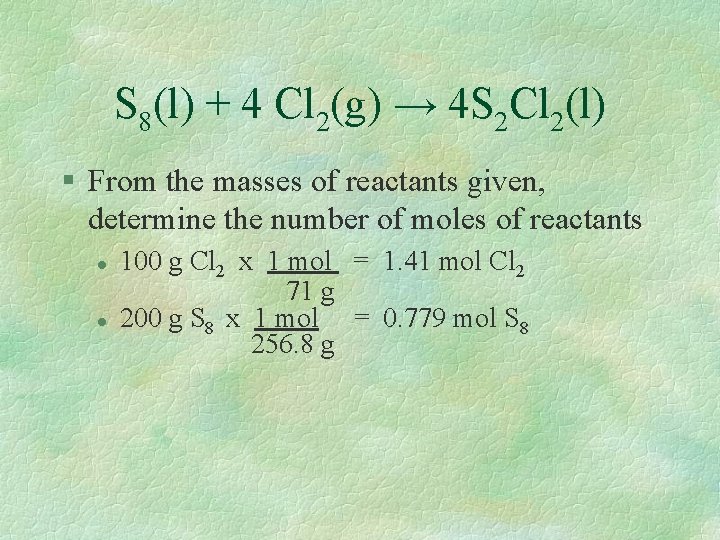



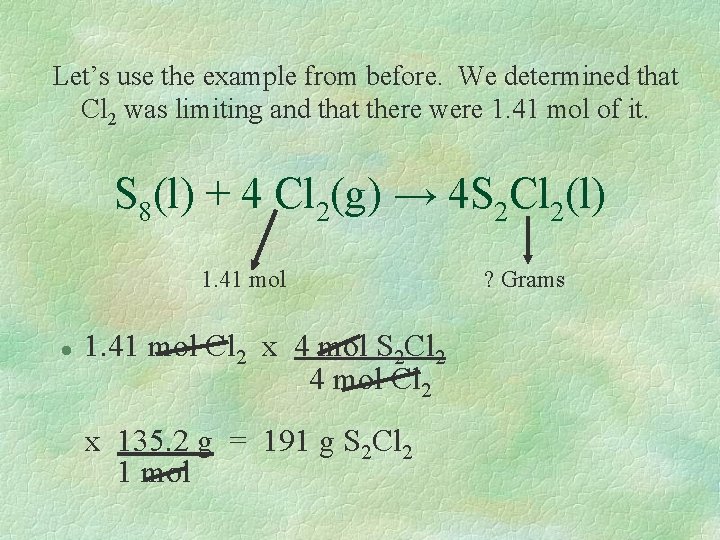


- Slides: 18

Chapter 11: Stoichiometry Sec. 11. 3: Limiting Reactants

Objectives § Identify the limiting reactant in a chemical equation. § Identify the excess reactant and calculate the amount remaining after the reaction is complete. § Calculate the mass of a product when the amounts of more than one reactant are given.

Limiting Reactants If I want to make s’mores, does it matter how many marshmallows I have if I only have one piece of chocolate? No!! I will use up the chocolate & there will be marshmallows left over!

Limiting Reactants – p. 379, Fig. 4 § If I have 10 screwdrivers, 5 pliers, & 4 hammers, how many tool sets could I make? § Each tool set consists of 2 screwdrivers, a pliers and a hammer. § The hammers are used up. The number of hammers limits how many sets can be made. § There are leftover pliers and screwdrivers. There is an excess of these tools.

In Chemical Reactions: § A reaction will proceed until one reactant is used up. § The amount of product formed depends upon the reactant that is limited. When this reactant is used up, the reaction stops. § The limiting reactant is the reactant that limits the extent of the reaction and determines the amount of product formed.

In Chemical Reactions: § When the limiting reactant is used up, a portion of all of the other reactants will remain after the reaction stops. § These left-over reactants are called excess reactants.

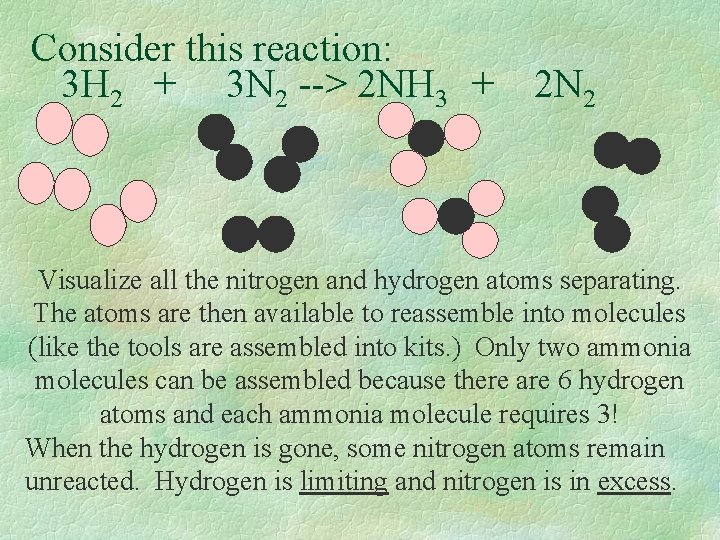
Consider this reaction: 3 H 2 + 3 N 2 --> 2 NH 3 + 2 N 2 Visualize all the nitrogen and hydrogen atoms separating. The atoms are then available to reassemble into molecules (like the tools are assembled into kits. ) Only two ammonia molecules can be assembled because there are 6 hydrogen atoms and each ammonia molecule requires 3! When the hydrogen is gone, some nitrogen atoms remain unreacted. Hydrogen is limiting and nitrogen is in excess.

Determining the limiting reactant & amount of product § If 200 g of sulfur reacts with 100 g of chlorine, what mass of disulfur dichloride is produced? S 8(l) + 4 Cl 2(g) → 4 S 2 Cl 2(l) Remember: The amount of product depends on the reactant that is limited. Finding that is your first step!
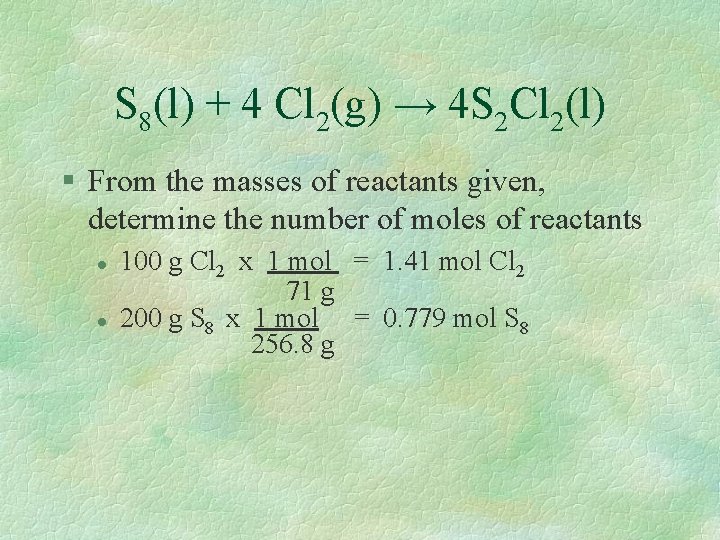
S 8(l) + 4 Cl 2(g) → 4 S 2 Cl 2(l) § From the masses of reactants given, determine the number of moles of reactants l l 100 g Cl 2 x 1 mol = 1. 41 mol Cl 2 71 g 200 g S 8 x 1 mol = 0. 779 mol S 8 256. 8 g

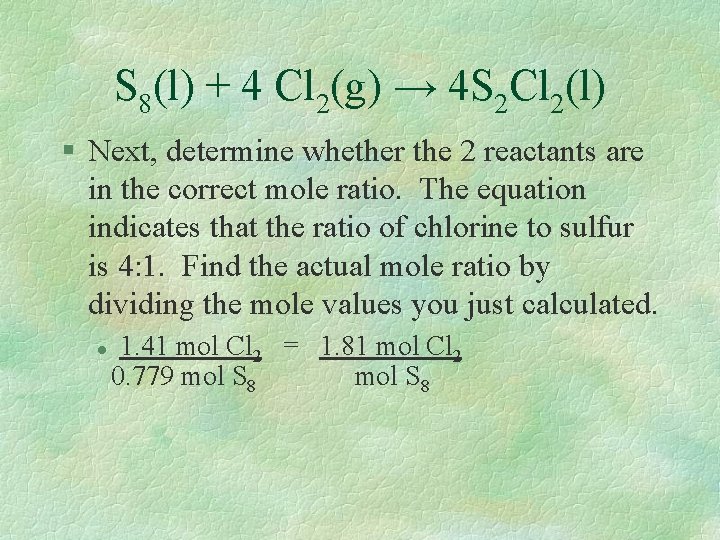
S 8(l) + 4 Cl 2(g) → 4 S 2 Cl 2(l) § Next, determine whether the 2 reactants are in the correct mole ratio. The equation indicates that the ratio of chlorine to sulfur is 4: 1. Find the actual mole ratio by dividing the mole values you just calculated. l 1. 41 mol Cl 2 = 1. 81 mol Cl 2 0. 779 mol S 8

The Limiting Reactant l l l This means that 1. 81 mol of chlorine is available for each mole of S 8. The mole ratio says we need 4 chlorine for 1 sulfur. There is not enough chlorine to use up all the sulfur so chlorine is the limiting reactant!!

Practice Problems § Identify the limiting reactant when 3. 50 g of HCl reacts with 5. 28 g of Na. OH to produce Na. Cl and water. § Identify the limiting reactant when 1. 22 g O 2 reacts with 1. 05 g H 2 to produce water.

Determining the amount of product § Since the limiting reactant determines the amount of product formed, the amount of product can be determined when the limiting reactant is known. § The moles of the limiting reactant will be our “known” in a standard stoichiometric calculation. The mass of the product is the unknown.
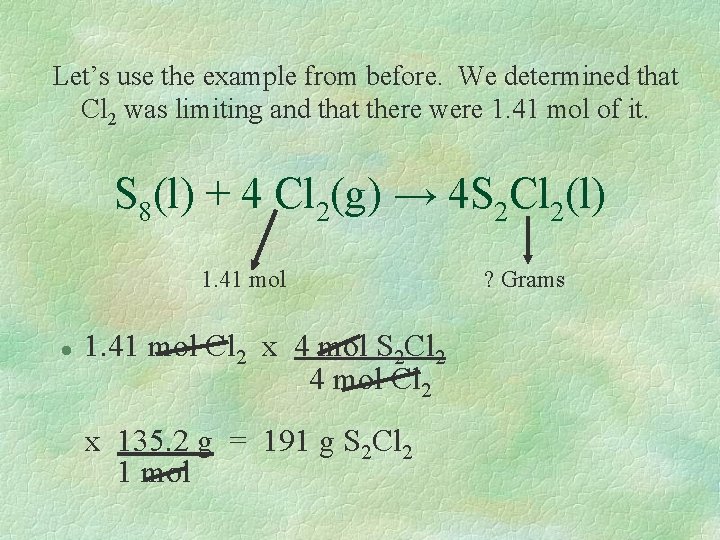
Let’s use the example from before. We determined that Cl 2 was limiting and that there were 1. 41 mol of it. S 8(l) + 4 Cl 2(g) → 4 S 2 Cl 2(l) 1. 41 mol l 1. 41 mol Cl 2 x 4 mol S 2 Cl 2 4 mol Cl 2 x 135. 2 g = 191 g S 2 Cl 2 1 mol ? Grams

Practice Problems § Determine the mass of tetraphosphorus decoxide formed if 25. 0 g of phosphorus (P 4) and 50. 0 g oxygen are combined. P 4 + 5 O 2 --> P 4 O 10 § In photosynthesis, 6 CO 2 + 6 H 2 O --> C 6 H 12 O 6 + 6 O 2. If a plant has 88. 0 g of CO 2 and 64. 0 g H 2 O available, what mass of glucose will be produced?

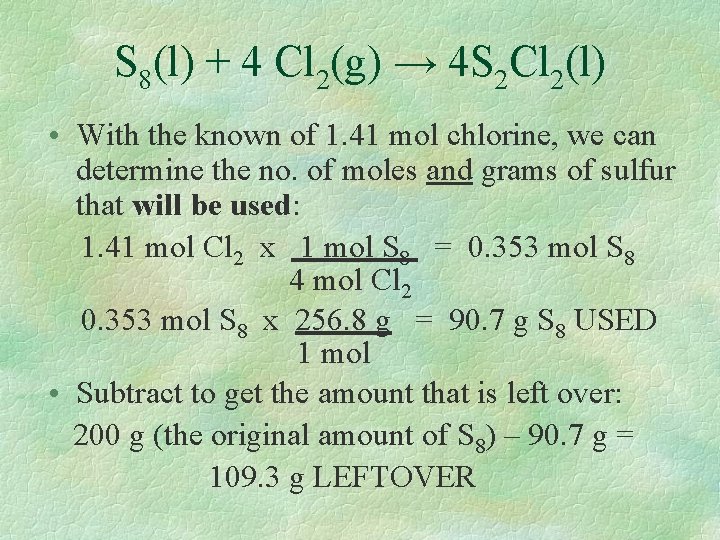
Excess Reactants § Once the limiting reactant has been determined, it is also possible to use stoichiometry to find out how much of the excess reactant is used or how much is leftover. § Recall this reaction: S 8(l) + 4 Cl 2(g) → 4 S 2 Cl 2(l) We determined the limiting reactant is chlorine, with the amount of 1. 41 moles.

S 8(l) + 4 Cl 2(g) → 4 S 2 Cl 2(l) • With the known of 1. 41 mol chlorine, we can determine the no. of moles and grams of sulfur that will be used: 1. 41 mol Cl 2 x 1 mol S 8 = 0. 353 mol S 8 4 mol Cl 2 0. 353 mol S 8 x 256. 8 g = 90. 7 g S 8 USED 1 mol • Subtract to get the amount that is left over: 200 g (the original amount of S 8) – 90. 7 g = 109. 3 g LEFTOVER

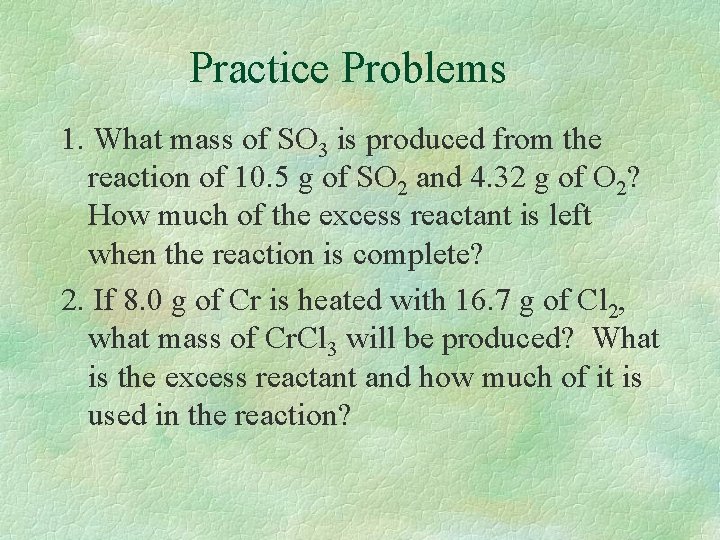
Practice Problems 1. What mass of SO 3 is produced from the reaction of 10. 5 g of SO 2 and 4. 32 g of O 2? How much of the excess reactant is left when the reaction is complete? 2. If 8. 0 g of Cr is heated with 16. 7 g of Cl 2, what mass of Cr. Cl 3 will be produced? What is the excess reactant and how much of it is used in the reaction?