Stoichiometry Chemical Quantities Notes Stoichiometry Study of quantitative

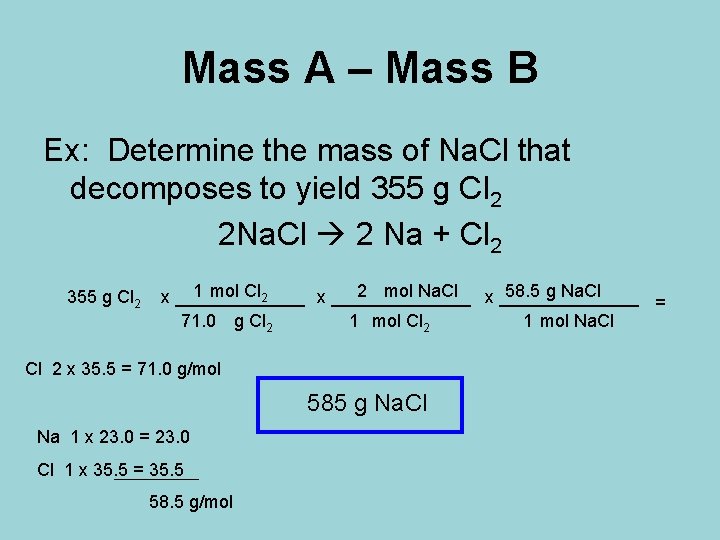
- Slides: 6

Stoichiometry – Chemical Quantities Notes

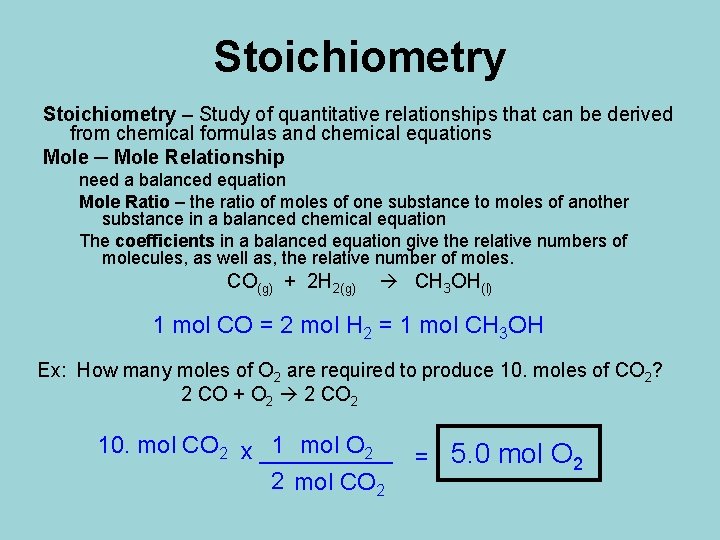
Stoichiometry – Study of quantitative relationships that can be derived from chemical formulas and chemical equations Mole ─ Mole Relationship need a balanced equation Mole Ratio – the ratio of moles of one substance to moles of another substance in a balanced chemical equation The coefficients in a balanced equation give the relative numbers of molecules, as well as, the relative number of moles. CO(g) + 2 H 2(g) CH 3 OH(l) 1 mol CO = 2 mol H 2 = 1 mol CH 3 OH Ex: How many moles of O 2 are required to produce 10. moles of CO 2? 2 CO + O 2 2 CO 2 10. mol CO 2 x _____ 1 mol O 2 = 5. 0 mol O 2 2 mol CO 2

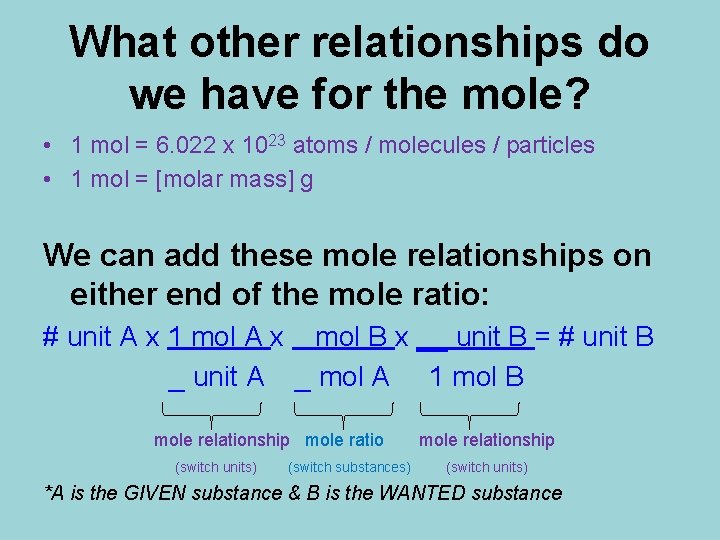
What other relationships do we have for the mole? • 1 mol = 6. 022 x 1023 atoms / molecules / particles • 1 mol = [molar mass] g We can add these mole relationships on either end of the mole ratio: # unit A x 1 mol A x mol B x __ unit B = # unit B _ unit A _ mol A 1 mol B mole relationship mole ratio (switch units) (switch substances) mole relationship (switch units) *A is the GIVEN substance & B is the WANTED substance

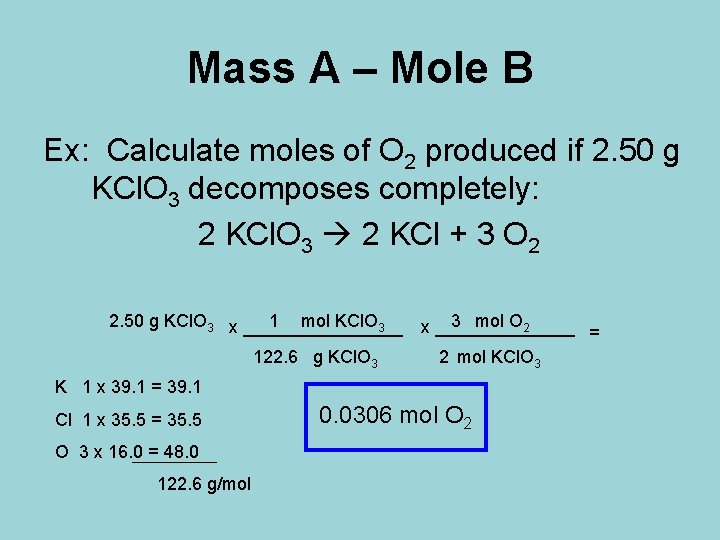
Mass A – Mole B Ex: Calculate moles of O 2 produced if 2. 50 g KCl. O 3 decomposes completely: 2 KCl. O 3 2 KCl + 3 O 2 2. 50 g KCl. O 3 x ________ 1 mol KCl. O 3 3 mol O 2 x _______ = 122. 6 g KCl. O 3 2 mol KCl. O 3 K 1 x 39. 1 = 39. 1 Cl 1 x 35. 5 = 35. 5 O 3 x 16. 0 = 48. 0 122. 6 g/mol 0. 0306 mol O 2
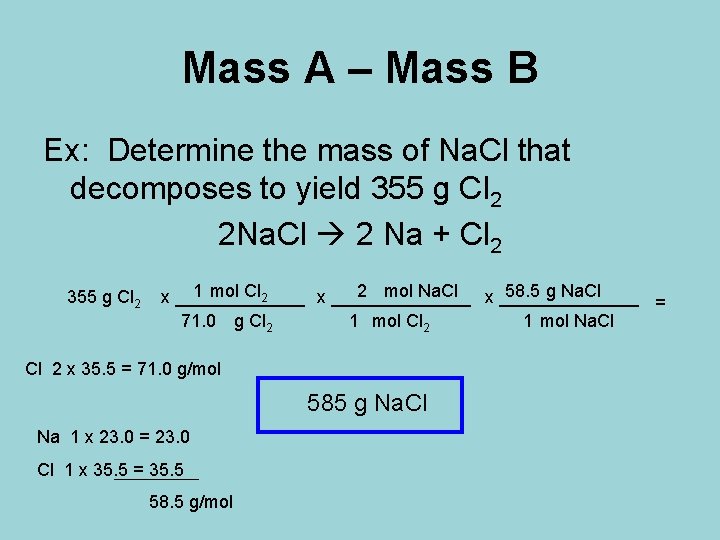
Mass A – Mass B Ex: Determine the mass of Na. Cl that decomposes to yield 355 g Cl 2 2 Na. Cl 2 Na + Cl 2 355 g Cl 2 1 mol Cl 2 2 mol Na. Cl 58. 5 g Na. Cl x ______________ x _______ = 71. 0 g Cl 2 1 mol Na. Cl Cl 2 x 35. 5 = 71. 0 g/mol 585 g Na. Cl Na 1 x 23. 0 = 23. 0 Cl 1 x 35. 5 = 35. 5 58. 5 g/mol

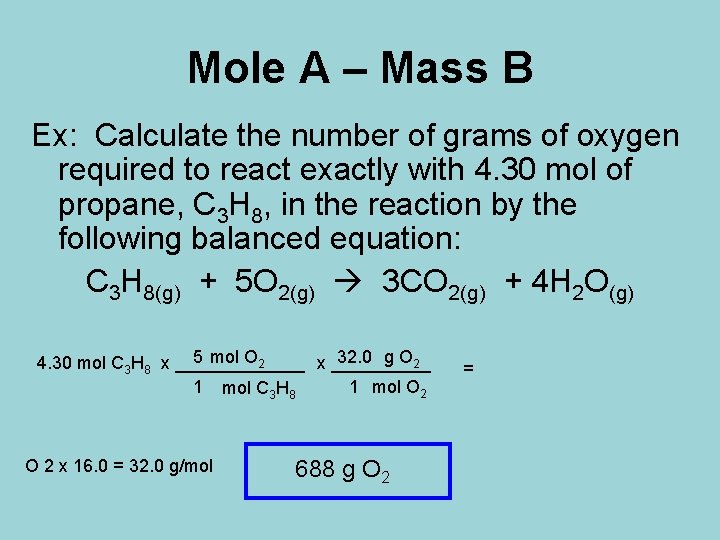
Mole A – Mass B Ex: Calculate the number of grams of oxygen required to react exactly with 4. 30 mol of propane, C 3 H 8, in the reaction by the following balanced equation: C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(g) 5 mol O 2 32. 0 g O 2 4. 30 mol C 3 H 8 x _______ x _____ 1 mol C 3 H 8 1 mol O 2 x 16. 0 = 32. 0 g/mol 688 g O 2 =