Chemistry Chapter 11 STOICHIOMETRY 11 1 Defining Stoichiometry














- Slides: 26

Chemistry Chapter 11 STOICHIOMETRY

11. 1 Defining Stoichiometry Objectives: 1. Describe the types of relationships indicated by a balanced chemical equation 2. State the mole ratios from a balanced chemical equation

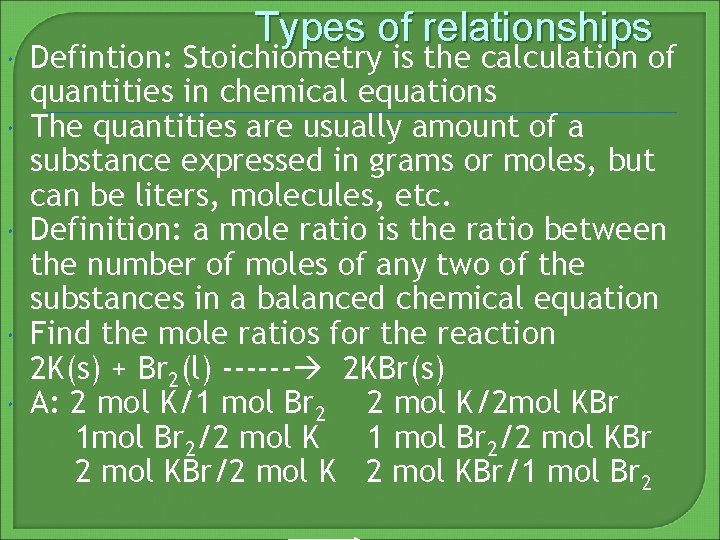
Types of relationships Defintion: Stoichiometry is the calculation of quantities in chemical equations The quantities are usually amount of a substance expressed in grams or moles, but can be liters, molecules, etc. Definition: a mole ratio is the ratio between the number of moles of any two of the substances in a balanced chemical equation Find the mole ratios for the reaction 2 K(s) + Br 2(l) ------ 2 KBr(s) A: 2 mol K/1 mol Br 2 2 mol K/2 mol KBr 1 mol Br 2/2 mol K 1 mol Br 2/2 mol KBr/2 mol KBr/1 mol Br 2

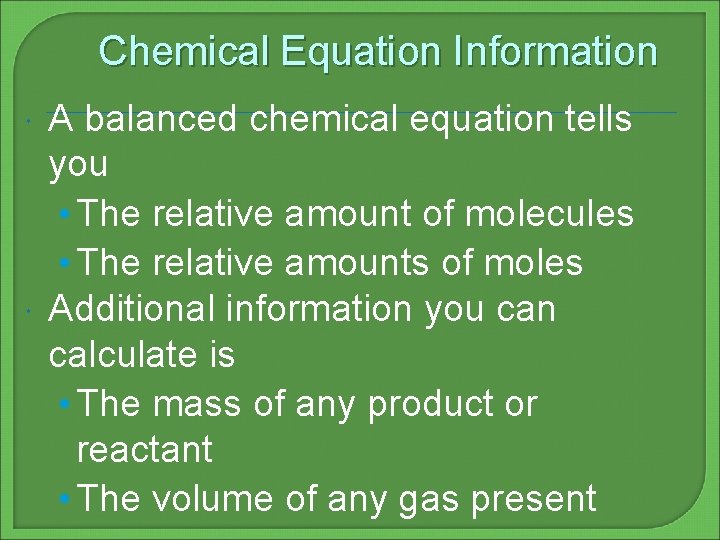
Chemical Equation Information A balanced chemical equation tells you • The relative amount of molecules • The relative amounts of moles Additional information you can calculate is • The mass of any product or reactant • The volume of any gas present

The coefficients in an equation can be interpreted on the micro or macro levels • Micro: how many representative units are reacting or produced • Macro: how many moles of each substance are present The coefficients in front of each chemical equals how many moles & how many molecules of each chemical there are A balanced chemical equation gives the ratio of moles of one chemical to moles of any other chemical in the equation

The number of moles can be used to make conversion factors. For example in the equation N 2(g) + 3 H 2(g) → 2 NH 3(g) 1 mol of nitrogen reacts with three moles of hydrogen to form two moles of ammonia Mass: you can calculate the mass of each product or reactant by multiplying the number of moles by the chemical’s molecular mass • Mass is conserved, so the mass of the products should equal the mass of the reactants

The volume of any gas present, if you assume STP conditions, can be found by using the molar volume definition • 1 mol = 22. 4 L The volume is simply the number of moles multiplied by (22. 4 L / 1 mol) Let’s try a problem: Interpret the combustion of propane in terms of representative particles, moles and conservation of mass

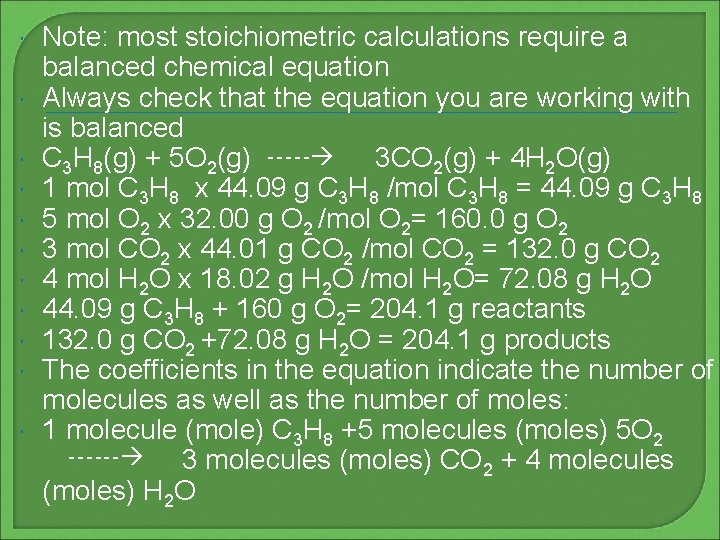
Note: most stoichiometric calculations require a balanced chemical equation Always check that the equation you are working with is balanced C 3 H 8(g) + 5 O 2(g) ----- 3 CO 2(g) + 4 H 2 O(g) 1 mol C 3 H 8 x 44. 09 g C 3 H 8 /mol C 3 H 8 = 44. 09 g C 3 H 8 5 mol O 2 x 32. 00 g O 2 /mol O 2= 160. 0 g O 2 3 mol CO 2 x 44. 01 g CO 2 /mol CO 2 = 132. 0 g CO 2 4 mol H 2 O x 18. 02 g H 2 O /mol H 2 O= 72. 08 g H 2 O 44. 09 g C 3 H 8 + 160 g O 2= 204. 1 g reactants 132. 0 g CO 2 +72. 08 g H 2 O = 204. 1 g products The coefficients in the equation indicate the number of molecules as well as the number of moles: 1 molecule (mole) C 3 H 8 +5 molecules (moles) 5 O 2 ------ 3 molecules (moles) CO 2 + 4 molecules (moles) H 2 O

11. 2 Stoichiometric Calculations Objectives: 1. List the sequence of steps used in solving stoichiometric problems 2. Solve stoichiometric problems

The Steps to Solving Mole ratios can then be used to find unknown quantities of reactants or products The general approach to solving for the unknown is to use the given amount of moles from the information in the problem, multiply it by the mole ratio and then calculate

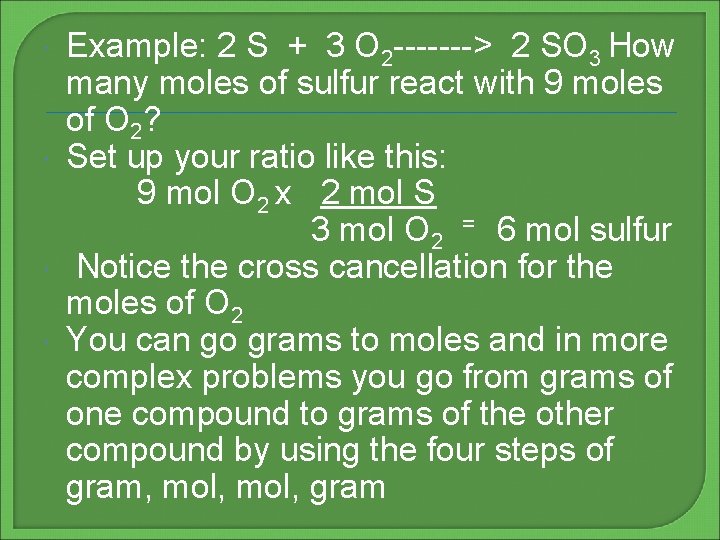
Example: 2 S + 3 O 2 -------> 2 SO 3 How many moles of sulfur react with 9 moles of O 2? Set up your ratio like this: 9 mol O 2 x 2 mol S 3 mol O 2 = 6 mol sulfur Notice the cross cancellation for the moles of O 2 You can go grams to moles and in more complex problems you go from grams of one compound to grams of the other compound by using the four steps of gram, mol, gram

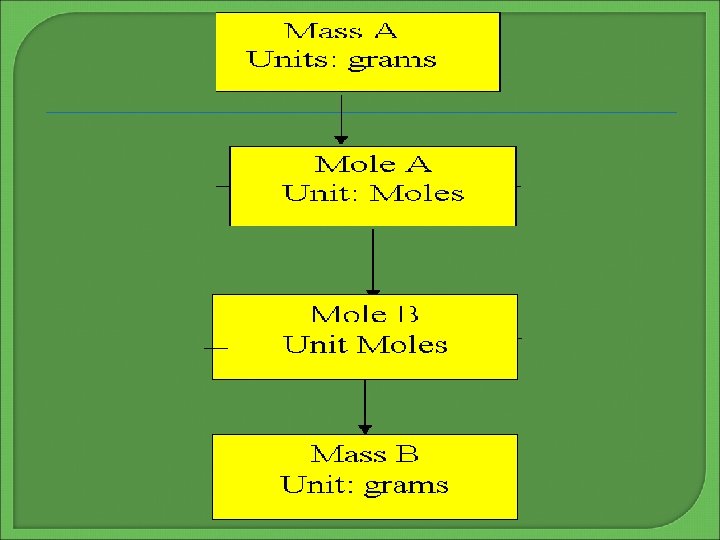
Example mole/mole 4 Al(s) + 3 O 2(g) → 2 Al 2 O 3(s) How many moles of aluminum are needed to form 3. 7 moles of Al 2 O 3(s)? The amount of substance is usually determined by finding its mass in grams Knowing the mass of one reactant or product in a balanced equation will let you determine the mass of any other reactant or product in the equation You cannot directly convert between products and reactants Use the gram, mol, gram calculations that follow this slide

An amount given in grams is first converted to moles using the molar mass of the given substance Then using the molar ratios from the balanced equation, convert the moles of given substance (which you just calculated) to the number of moles of the unknown using the principles of cross-cancellation Finally, convert to grams using the molar mass of the unknown The unknown can be either a reactant or a product To go from the mass of the given to the unknown use the four steps that follow

Steps to solving gram to gram problems: 1. Write down what you know (the givens, including proper units) 2. Convert to moles using molar mass(always include proper units) 3. Use the balanced equation to convert to moles of the unknown quantity (the desired quantity-keep using units) 4. convert back to grams using molar mass


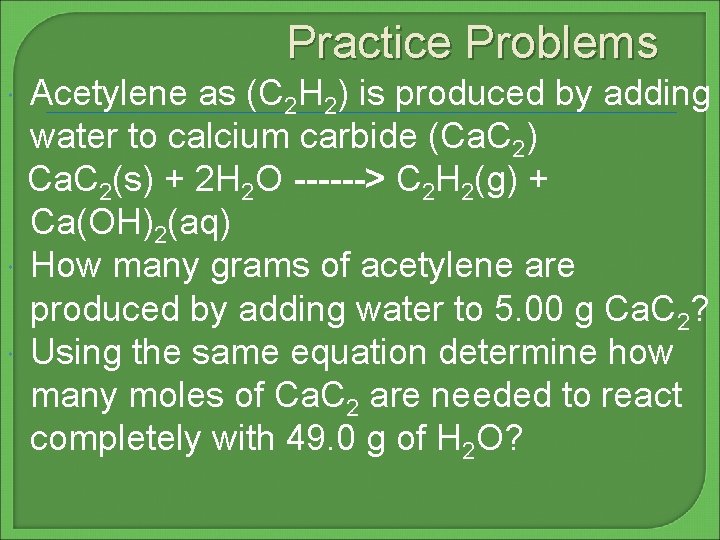
Practice Problems Acetylene as (C 2 H 2) is produced by adding water to calcium carbide (Ca. C 2) Ca. C 2(s) + 2 H 2 O ------> C 2 H 2(g) + Ca(OH)2(aq) How many grams of acetylene are produced by adding water to 5. 00 g Ca. C 2? Using the same equation determine how many moles of Ca. C 2 are needed to react completely with 49. 0 g of H 2 O?

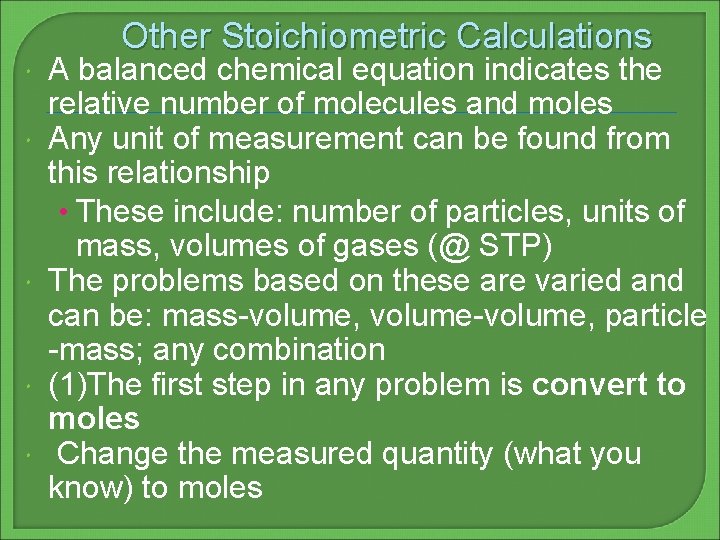
Other Stoichiometric Calculations A balanced chemical equation indicates the relative number of molecules and moles Any unit of measurement can be found from this relationship • These include: number of particles, units of mass, volumes of gases (@ STP) The problems based on these are varied and can be: mass-volume, volume-volume, particle -mass; any combination (1)The first step in any problem is convert to moles Change the measured quantity (what you know) to moles

(2) The second step is to use the mole ratio (from the balanced eqn) of wanted to given substances (3) Change moles of the wanted substance to a measured quantity (what you are looking for) How many molecules of oxygen are produced by the decomposition of 6. 54 g of potassium chlorate? 2 KCl. O 3 (s) ------> 2 KCl (s) + 3 O 2 (g)

11. 3 Limiting Reactants Objectives: 1. Identify the limiting reactant in a chemical equation 2. Identify the excess reactant, and calculate the remaining after the reaction is complete 3. Calculate the mass of a product when the amount of more than one reactant are given

Identifying the LR Chemicals in a reaction are required according to the coefficients in the balanced chemical equation Definition: a limiting reactant limits the extent of the reaction and therefore, the amount of product formed The chemical that gets used up first is called the limiting reactant or reagent It limits the amount of products that can be formed When the reaction stops, the limiting reactant will be all used up Definition: excess reactant are left over when a reaction stops

The known amount of one of the reactant is multiplied by the mole ratio (from the balanced equation) to calculate the required amount of the other reactant The required amount is compared to the given amount to see if it is limiting or not Sodium chloride can be prepared by the reaction of sodium metal with chlorine gas. Suppose 6. 7 mol Na reacts with 2. 3 mol Cl 2. What is the limiting reagent? How many moles of product are produced? 2 Na + Cl 2 --- 2 Na. Cl

Practice Determining the LR The reaction between solid white phosphorus (P 4) and oxygen produces tetraphosphorus decoxide (P 4 O 10). A. Determine the mass of P 4 O 10 formed if 25 g of P 4 and 50 g of oxygen are combined/ B. How much excess reactant remains afterward? 1. givens: mass of 25 g of P 4 and 50 g of oxygen 2. write the equation: P 4(s) + 5 O 2(g) - P 4 O 10(s) 3. What are you looking for? 4. How are you going to get there? Solve it!!

11. 4 Percent Yield Objectives: 1. Calculate theoretical yield of a chemical reaction from data 2. Determine the percent yield for a chemical reaction

Calculate Usually chemical reactions do not produce the quantity of product you would expect Definition: theoretical yield is the maximum amount of product that can produced from a given amount of reactant The amount of product expected is referred to as theoretical yield Its value comes from the balanced chemical equation Definition: the amount of product that is formed from performing the reaction (the experiment) is the actual yield

The measure of how close the actual yield is to theoretical yield is the percent yield The equation: percent yield equals actual yield divided by theoretical yield times 100 Percent yield is always a number less than 100 Percent yield is a measure of how efficient a reaction is in producing the desired product Reasons for low percent yield include: competing side reactions, impure reactants, improper procedures, improper measurements

Determine 1. Calcium carbonate decomposes by the reaction below. What is theoretical yield of Ca. O if 24. 8 g of Ca. CO 3 is heated? 2. What is the percent yield if 13. 1 g of Ca. O is formed? Ca. CO 3 --- Ca. O + CO 2 3. Determine theoretical yield of silver chromate if 0. 5 g of sliver nitrate reacts from the following equation: 2 Ag. NO 3(aq) + K 2 Cr. O 4(aq) - Ag 2 Cr. O 4(s) + 2 KNO 3(aq) 4. What is the percent yield if the reaction yields 0. 455 g Ag 2 Cr. O 4?