Stoichiometry Derived from the Greek roots stoicheion element
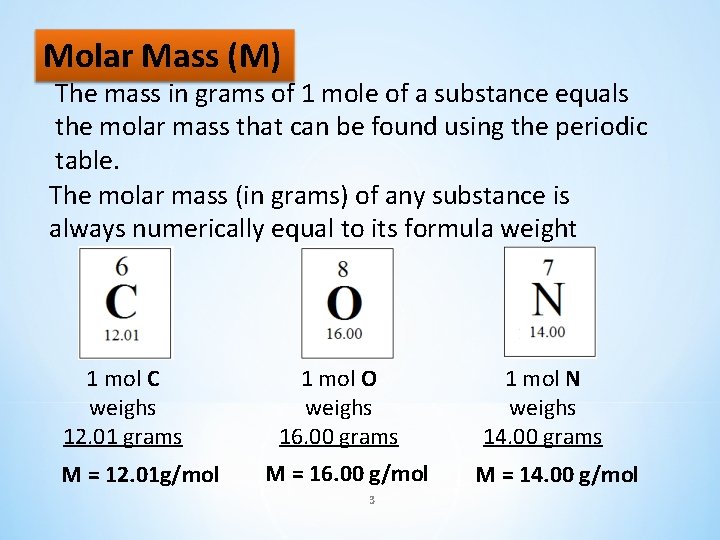











![Q) Urea [(NH 2)2 CO] is produced by reacting ammonia with carbon dioxide. For Q) Urea [(NH 2)2 CO] is produced by reacting ammonia with carbon dioxide. For](https://slidetodoc.com/presentation_image_h2/e683762aaf453cf477034d7867156809/image-43.jpg)














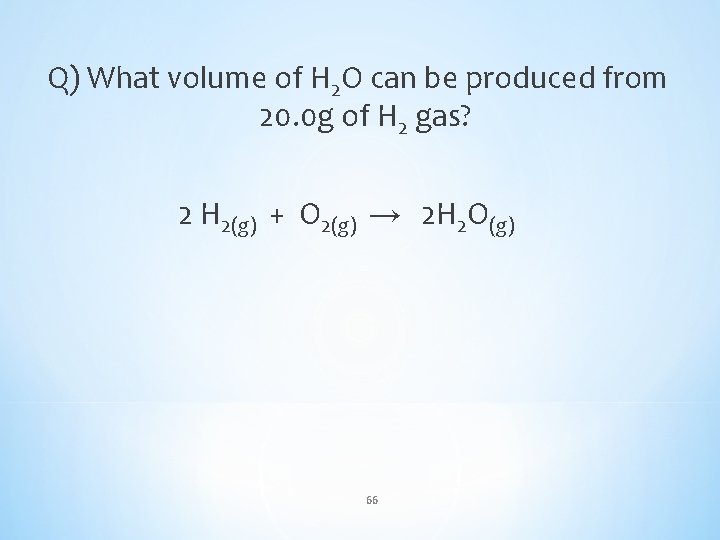


- Slides: 69

Stoichiometry Derived from the Greek roots "stoicheion" = element & “metron” = measure the analysis of elements the ratios in which they combine how they can interact to form something new 1

Mole The most common unit of measurement in chemistry It is used to measure atoms and molecules 1 mole (mol) = 6. 02 • 1023 1 mole of substance contains this many particles 6. 02 • 1023 is known as Avogadro’s Number Memorize this number! 2
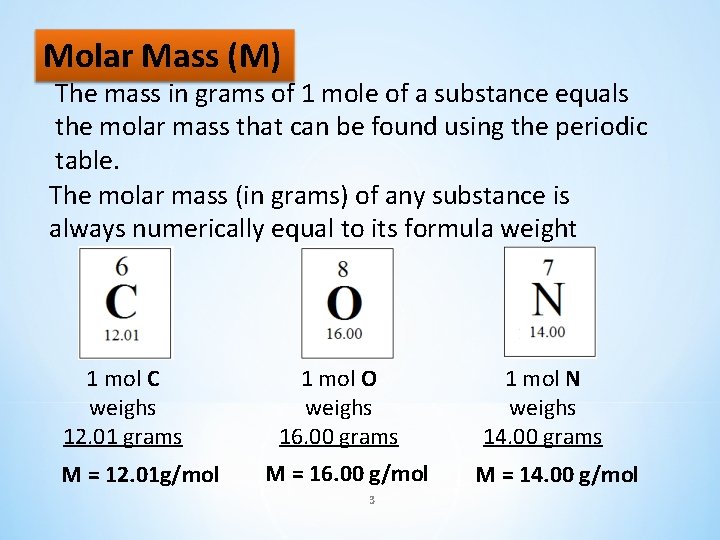
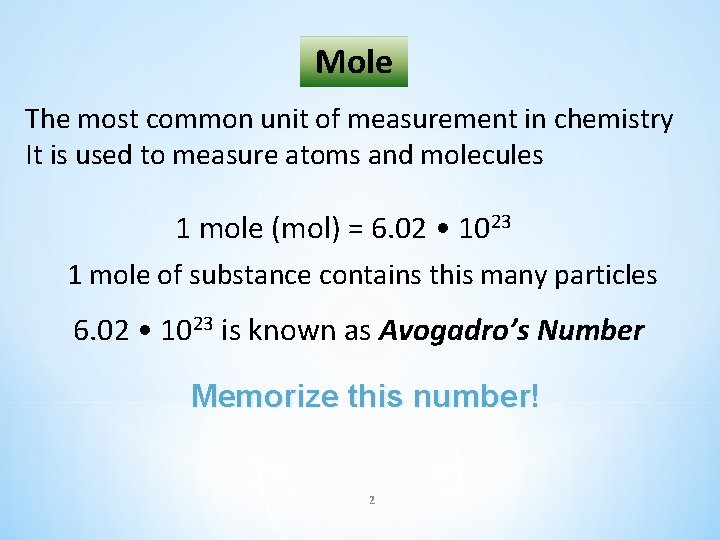
Molar Mass (M) The mass in grams of 1 mole of a substance equals the molar mass that can be found using the periodic table. The molar mass (in grams) of any substance is always numerically equal to its formula weight 1 mol C weighs 12. 01 grams M = 12. 01 g/mol 1 mol O weighs 16. 00 grams M = 16. 00 g/mol 3 1 mol N weighs 14. 00 grams M = 14. 00 g/mol

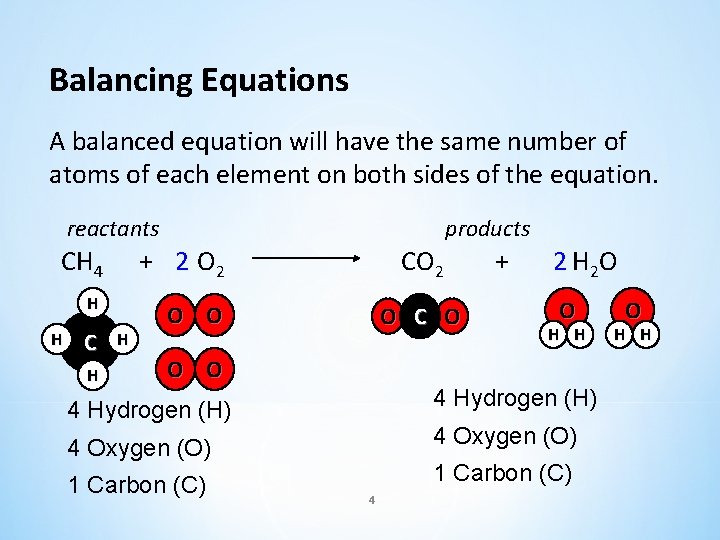
Balancing Equations A balanced equation will have the same number of atoms of each element on both sides of the equation. reactants products CH 4 + 2 O 2 H O O H C H H CO 2 O C O + 2 H 2 O O H H O O 4 Hydrogen (H) 4 Oxygen (O) 1 Carbon (C) 4 O H H

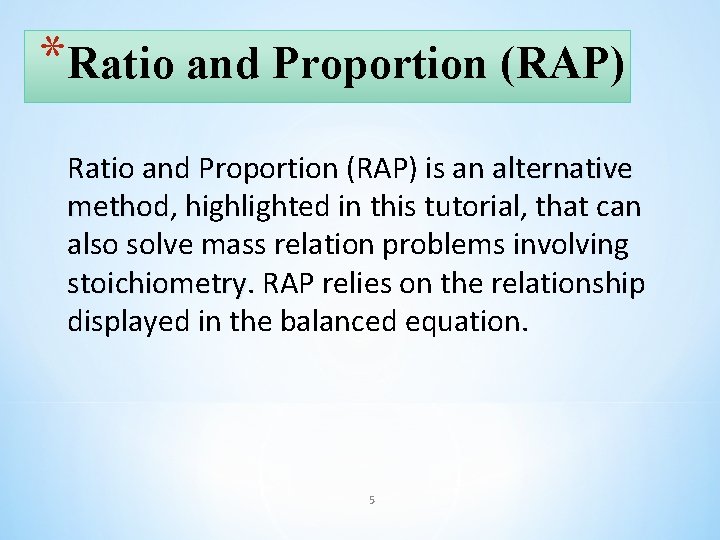
*Ratio and Proportion (RAP) is an alternative method, method highlighted in this tutorial, that can also solve mass relation problems involving stoichiometry RAP relies on the relationship displayed in the balanced equation. 5

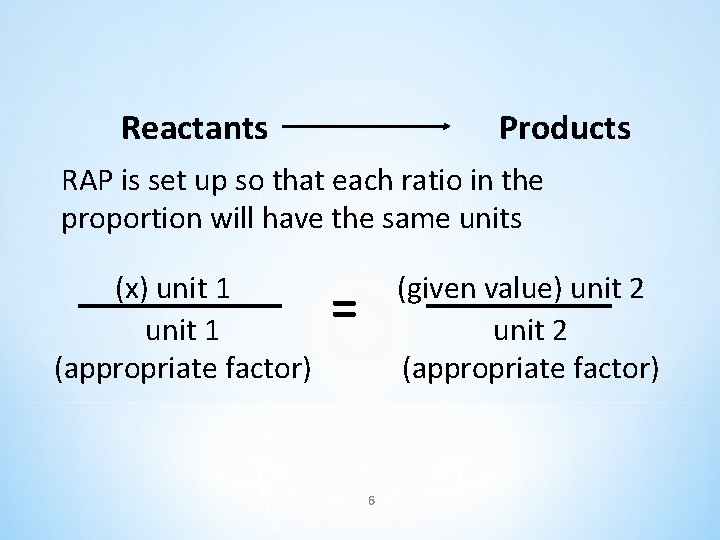
Reactants Products RAP is set up so that each ratio in the proportion will have the same units (x) unit 1 (appropriate factor) (given value) unit 2 (appropriate factor) = 6

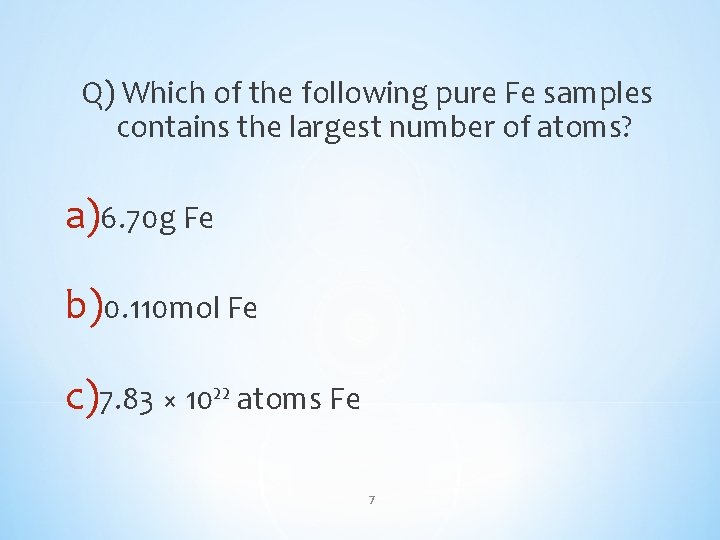
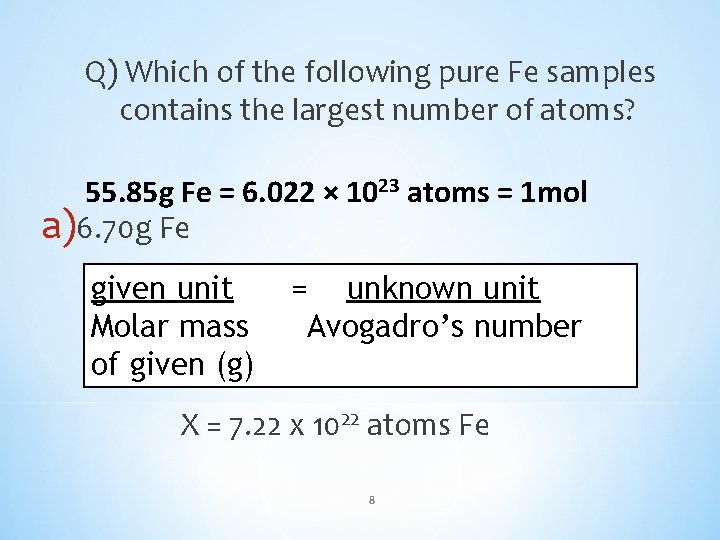
Q) Which of the following pure Fe samples contains the largest number of atoms? a)6. 70 g Fe b)0. 110 mol Fe c)7. 83 × 10²² atoms Fe 7

Q) Which of the following pure Fe samples contains the largest number of atoms? 55. 85 g Fe = 6. 022 × 1023 atoms = 1 mol a)6. 70 g Fe 6. 70 gunit Fe = = X unknown (atoms Fe)unit given 55. 85 gmass Fe 6. 022 x 1023 atoms Fe Molar Avogadro’s number of given (g) X = 7. 22 x 1022 atoms Fe 8

Q) Which of the following pure Fe samples contains the largest number of atoms? 55. 85 g Fe = 6. 022 × 1023 atoms = 1 mol b) 0. 110 mol Fe 0. 110 Fe == x (atoms Fe) unit givenmol unit unknown 1 Moles mol Feof 6. 022 x 1023 atoms Fe Avogadro’s number given (mol) x = 6. 6 x 1022 atoms Fe 9

Q) Which of the following pure Fe samples contains the largest number of atoms? 55. 85 g Fe = 6. 022 × 1023 atoms = 1 mol c) 7. 83 × 10²² atoms Fe It’s already in atoms! 10

Q) Which of the following pure Fe samples contains the largest number of atoms? a) 7. 22 × 10²² atoms Fe b) 6. 6 × 10²² atoms Fe c) 7. 83 × 10²² atoms Fe 11

*Set it up: 85 mol Sn = x atoms Sn 1 mol Sn 6. 02 x 1023 atoms Sn (85 mol Sn)(6. 02 x 1023 atoms Sn) = (x)(1 mol Sn) x = 5. 117 x 1025 atoms Sn = 5. 1 x 1025 atoms Sn *

*First, consider the number of moles of H in one water molecule…. 2 *Set up the problem with correct number of moles for H 1. 1 mol H 2 O = x atoms H 1 mole H 2 O 2(6. 02 x 1023) atoms H x = 1. 3244 x 1024 atoms H = 1. 3 x 1024 atoms H *

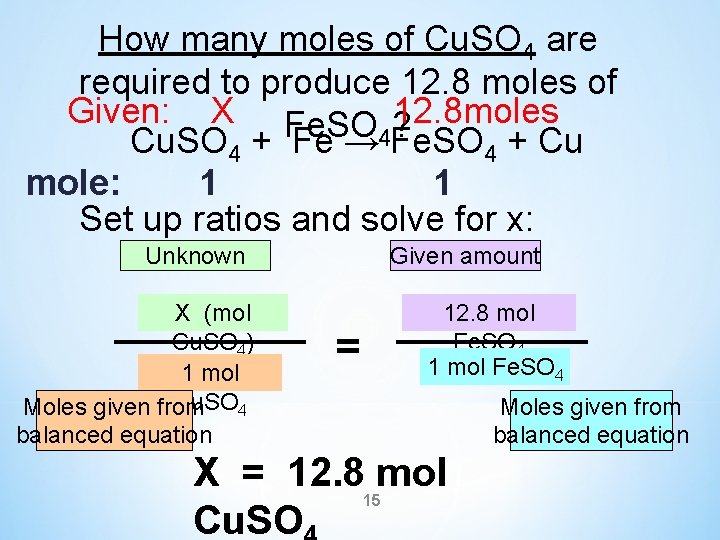
How many moles of Cu. SO 4 are required to produce 12. 8 moles of Fe. SO 4? Cu. SO 4 + Fe → Fe. SO 4 + Cu 14

How many moles of Cu. SO 4 are required to produce 12. 8 moles of Given: X Fe. SO ? 12. 8 moles Cu. SO + Fe → 4 Fe. SO + Cu 4 4 mole: 1 1 Set up ratios and solve for x: Unknown X (mol Cu. SO 4) 1 mol Cu. SO 4 Moles given from balanced equation Given amount 12. 8 mol Fe. SO 4 1 mol Fe. SO 4 = Moles given from balanced equation X = 12. 8 mol Cu. SO 15

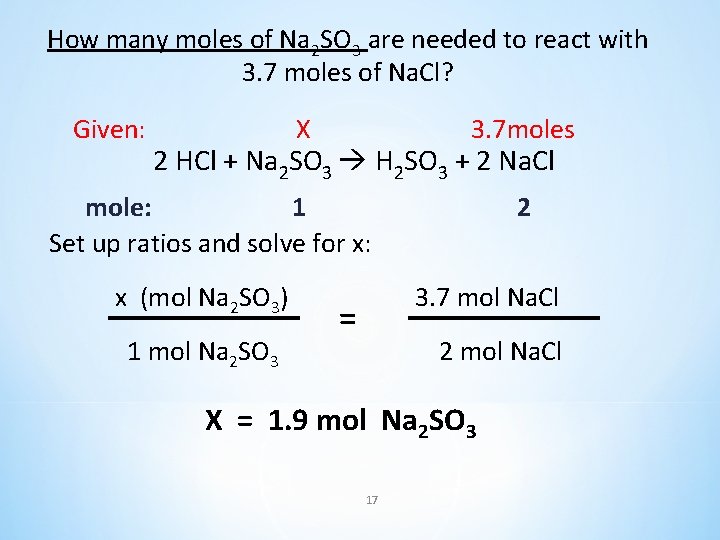
How many moles of Na 2 SO 3 are needed to produce with 3. 7 moles of Na. Cl? 2 HCl + Na 2 SO 3 H 2 SO 3 + 2 Na. Cl 16

How many moles of Na 2 SO 3 are needed to react with 3. 7 moles of Na. Cl? Given: X 3. 7 moles 2 HCl + Na 2 SO 3 H 2 SO 3 + 2 Na. Cl mole: 1 Set up ratios and solve for x: x (mol Na 2 SO 3) 1 mol Na 2 SO 3 2 3. 7 mol Na. Cl = 2 mol Na. Cl X = 1. 9 mol Na 2 SO 3 17

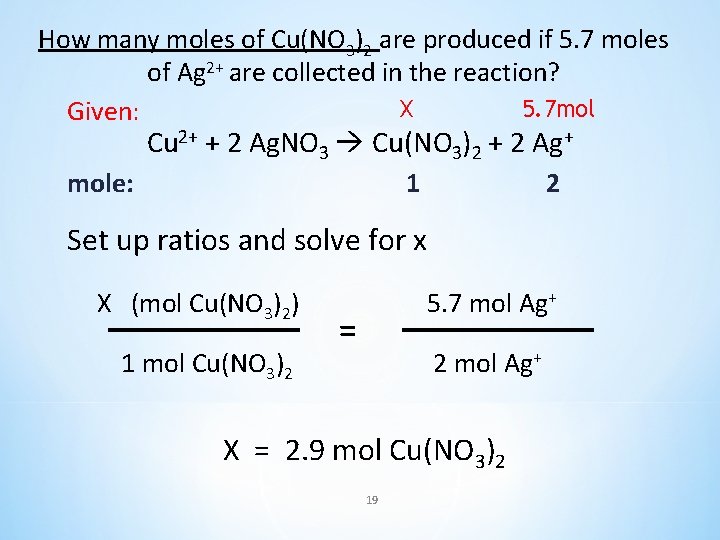
How many moles of Cu(NO 3)2 are produced if 5. 7 moles of Ag 2+ are collected in the reaction? Cu 2+ + 2 Ag. NO 3 Cu(NO 3)2 + 2 Ag+ 18

How many moles of Cu(NO 3)2 are produced if 5. 7 moles of Ag 2+ are collected in the reaction? X 5. 7 mol Given: Cu 2+ + 2 Ag. NO 3 Cu(NO 3)2 + 2 Ag+ mole: 1 2 Set up ratios and solve for x X (mol Cu(NO 3)2) 1 mol Cu(NO 3)2 5. 7 mol Ag+ = 2 mol Ag+ X = 2. 9 mol Cu(NO 3)2 19

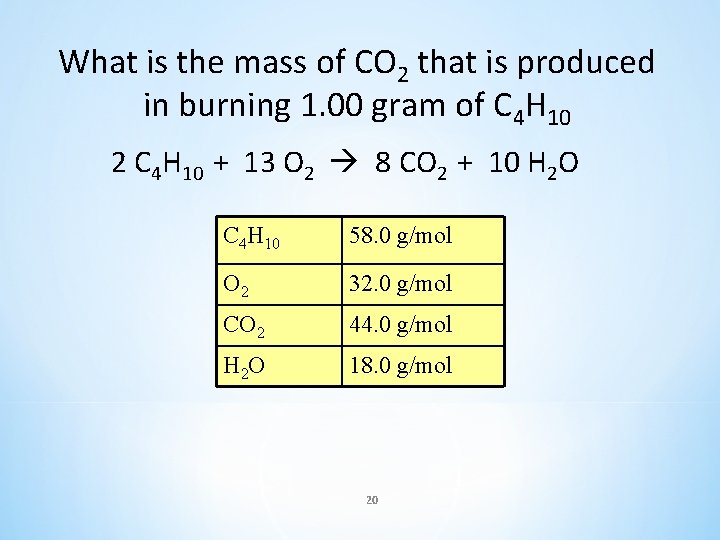
What is the mass of CO 2 that is produced in burning 1. 00 gram of C 4 H 10 2 C 4 H 10 + 13 O 2 8 CO 2 + 10 H 2 O C 4 H 10 58. 0 g/mol O 2 32. 0 g/mol CO 2 44. 0 g/mol H 2 O 18. 0 g/mol 20

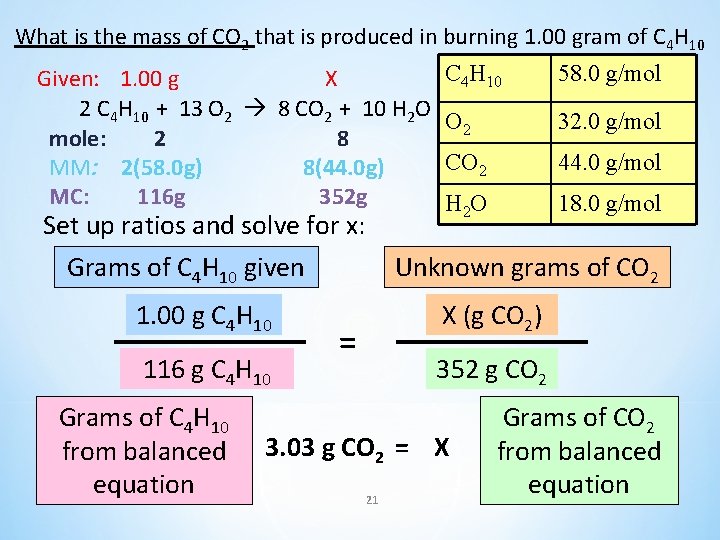
What is the mass of CO 2 that is produced in burning 1. 00 gram of C 4 H 10 Given: 1. 00 g X 2 C 4 H 10 + 13 O 2 8 CO 2 + 10 H 2 O mole: 2 8 MM: 2(58. 0 g) 8(44. 0 g) MC: 116 g 352 g Set up ratios and solve for x: Grams of C 4 H 10 given 1. 00 g C 4 H 10 116 g C 4 H 10 Grams of C 4 H 10 from balanced equation C 4 H 10 58. 0 g/mol O 2 32. 0 g/mol CO 2 44. 0 g/mol H 2 O 18. 0 g/mol Unknown grams of CO 2 X (g CO 2) = 352 g CO 2 3. 03 g CO 2 = X 21 Grams of CO 2 from balanced equation

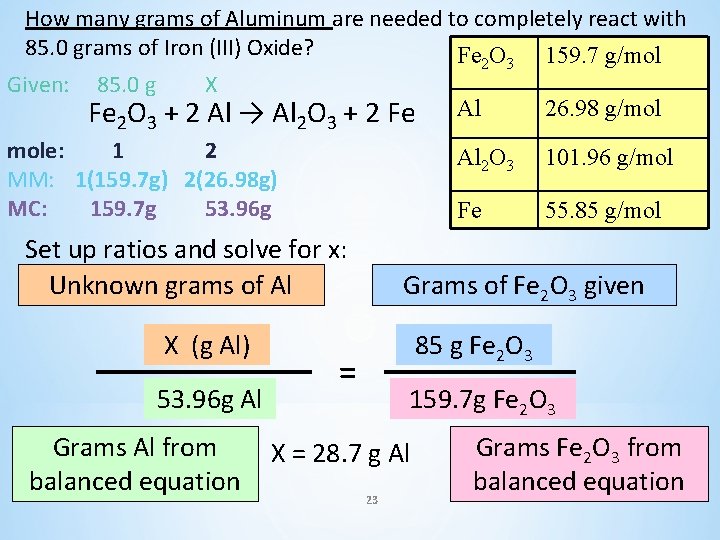
How many grams of Aluminum are needed to completely react with 85. 0 grams of Iron (III) Oxide? Fe 2 O 3 + 2 Al → Al 2 O 3 + 2 Fe Fe 2 O 3 160 g/mol Al 27. 0 g/mol Al 2 O 3 102. 0 g/mol Fe 112. 0 g/mol 22

How many grams of Aluminum are needed to completely react with 85. 0 grams of Iron (III) Oxide? Fe O 159. 7 g/mol Given: 85. 0 g 2 X Fe 2 O 3 + 2 Al → Al 2 O 3 + 2 Fe mole: 1 2 MM: 1(159. 7 g) 2(26. 98 g) MC: 159. 7 g 53. 96 g Set up ratios and solve for x: Unknown grams of Al X (g Al) 53. 96 g Al Grams Al from balanced equation 3 Al 26. 98 g/mol Al 2 O 3 101. 96 g/mol Fe 55. 85 g/mol Grams of Fe 2 O 3 given 85 g Fe 2 O 3 = 159. 7 g Fe 2 O 3 X = 28. 7 g Al 23 Grams Fe 2 O 3 from balanced equation

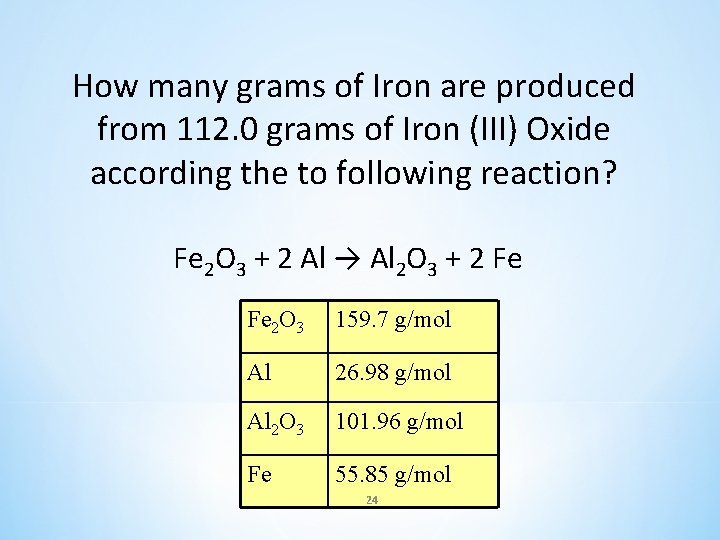
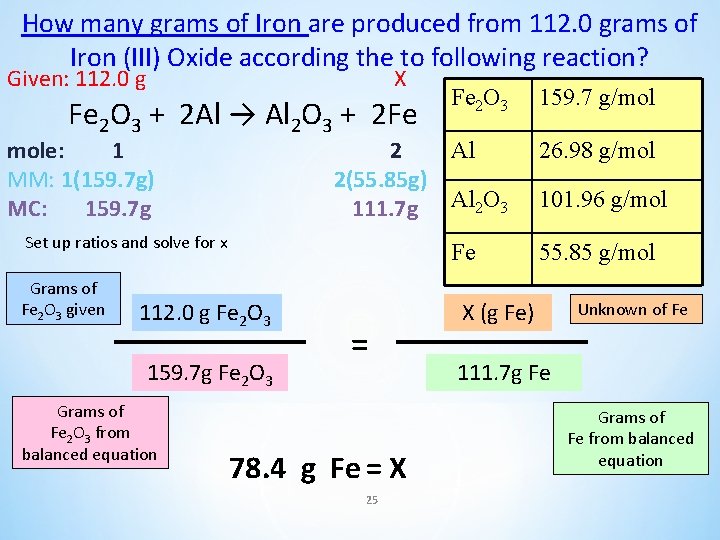
How many grams of Iron are produced from 112. 0 grams of Iron (III) Oxide according the to following reaction? Fe 2 O 3 + 2 Al → Al 2 O 3 + 2 Fe Fe 2 O 3 159. 7 g/mol Al 26. 98 g/mol Al 2 O 3 101. 96 g/mol Fe 55. 85 g/mol 24

How many grams of Iron are produced from 112. 0 grams of Iron (III) Oxide according the to following reaction? Given: 112. 0 g X Fe 2 O 3 + 2 Al → Al 2 O 3 + 2 Fe mole: 1 MM: 1(159. 7 g) MC: 159. 7 g 2 2(55. 85 g) 111. 7 g Set up ratios and solve for x Grams of Fe 2 O 3 given 112. 0 g Fe 2 O 3 159. 7 g Fe 2 O 3 Grams of Fe 2 O 3 from balanced equation = 78. 4 g Fe = X 25 Fe 2 O 3 159. 7 g/mol Al 26. 98 g/mol Al 2 O 3 101. 96 g/mol Fe 55. 85 g/mol X (g Fe) Unknown of Fe 111. 7 g Fe Grams of Fe from balanced equation

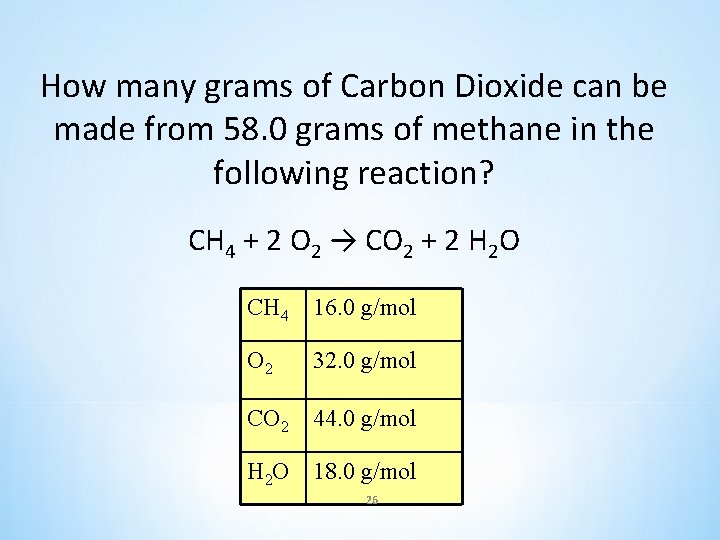
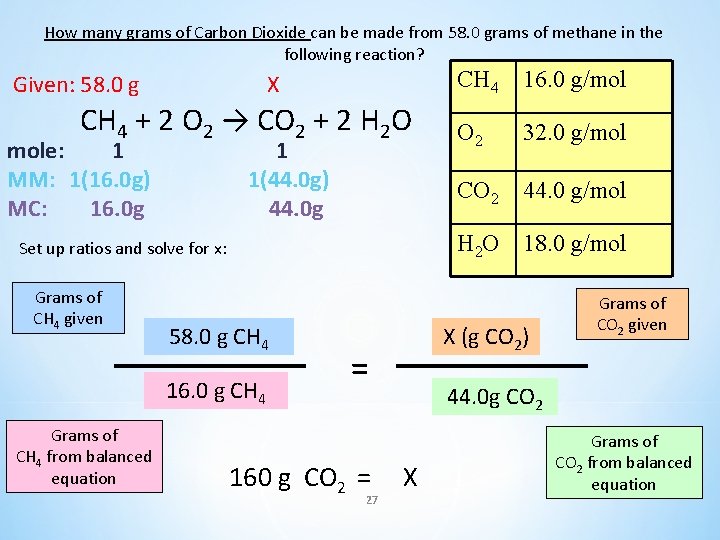
How many grams of Carbon Dioxide can be made from 58. 0 grams of methane in the following reaction? CH 4 + 2 O 2 → CO 2 + 2 H 2 O CH 4 16. 0 g/mol O 2 32. 0 g/mol CO 2 44. 0 g/mol H 2 O 18. 0 g/mol 26

How many grams of Carbon Dioxide can be made from 58. 0 grams of methane in the following reaction? Given: 58. 0 g X CH 4 + 2 O 2 → CO 2 + 2 H 2 O mole: 1 MM: 1(16. 0 g) MC: 16. 0 g 1 1(44. 0 g) 44. 0 g 58. 0 g CH 4 16. 0 g CH 4 Grams of CH 4 from balanced equation 16. 0 g/mol O 2 32. 0 g/mol CO 2 44. 0 g/mol H 2 O 18. 0 g/mol Set up ratios and solve for x: Grams of CH 4 given CH 4 X (g CO 2) = 160 g CO 2 = 27 Grams of CO 2 given 44. 0 g CO 2 X Grams of CO 2 from balanced equation

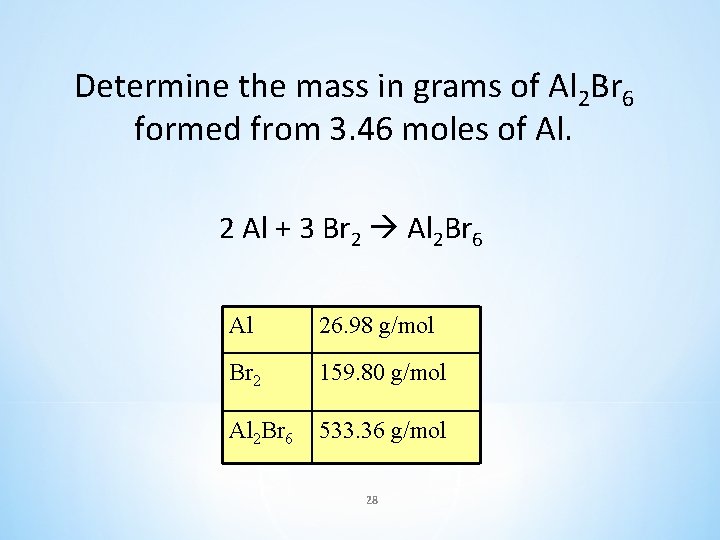
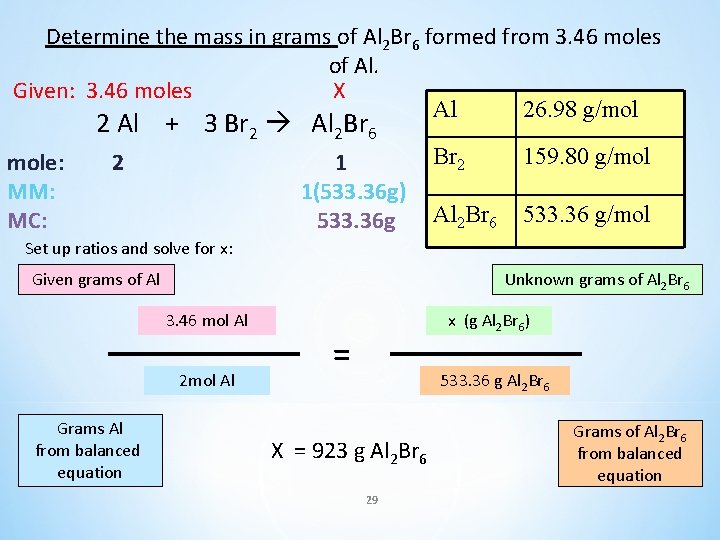
Determine the mass in grams of Al 2 Br 6 formed from 3. 46 moles of Al. 2 Al + 3 Br 2 Al 2 Br 6 Al 26. 98 g/mol Br 2 159. 80 g/mol Al 2 Br 6 533. 36 g/mol 28

Determine the mass in grams of Al 2 Br 6 formed from 3. 46 moles of Al. Given: 3. 46 moles X Al 26. 98 g/mol 2 Al + 3 Br 2 Al 2 Br 6 mole: MM: MC: 2 1 1(533. 36 g) 533. 36 g Br 2 159. 80 g/mol Al 2 Br 6 533. 36 g/mol Set up ratios and solve for x: Given grams of Al Unknown grams of Al 2 Br 6 3. 46 mol Al 2 mol Al Grams Al from balanced equation x (g Al 2 Br 6) = 533. 36 g Al 2 Br 6 X = 923 g Al 2 Br 6 29 Grams of Al 2 Br 6 from balanced equation

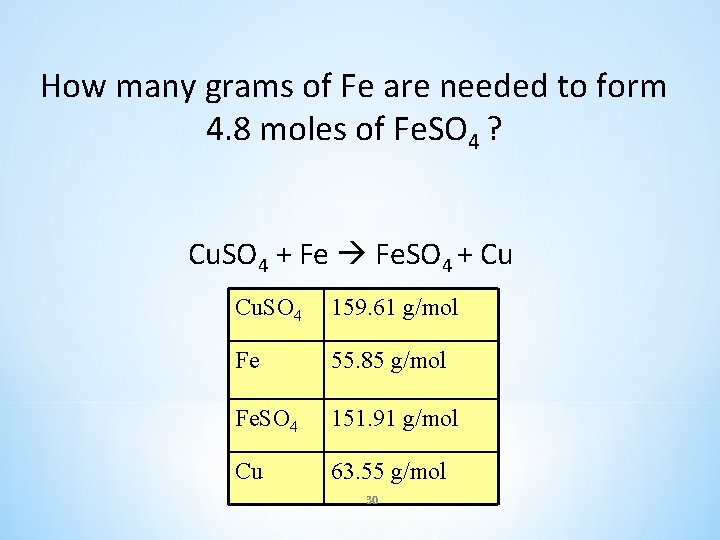
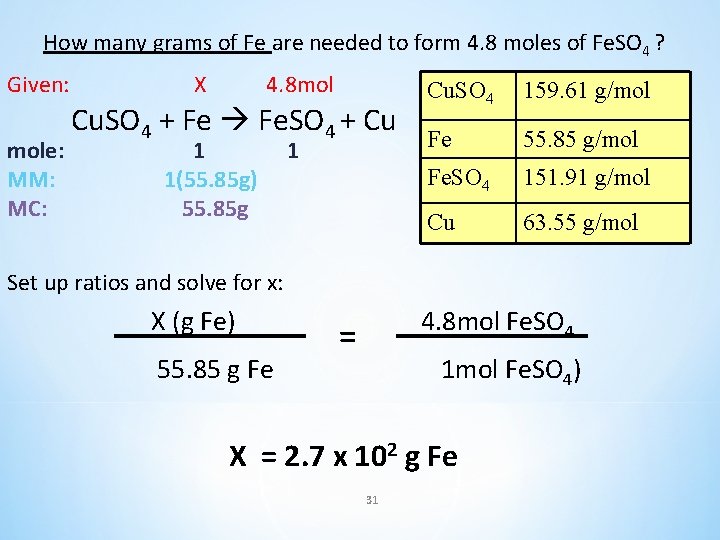
How many grams of Fe are needed to form 4. 8 moles of Fe. SO 4 ? Cu. SO 4 + Fe Fe. SO 4 + Cu Cu. SO 4 159. 61 g/mol Fe 55. 85 g/mol Fe. SO 4 151. 91 g/mol Cu 63. 55 g/mol 30

How many grams of Fe are needed to form 4. 8 moles of Fe. SO 4 ? Given: mole: MM: MC: X 4. 8 mol Cu. SO 4 + Fe Fe. SO 4 + Cu 1 1(55. 85 g) 55. 85 g 1 Cu. SO 4 159. 61 g/mol Fe 55. 85 g/mol Fe. SO 4 151. 91 g/mol Cu 63. 55 g/mol Set up ratios and solve for x: X (g Fe) 55. 85 g Fe 4. 8 mol Fe. SO 4 = 1 mol Fe. SO 4) X = 2. 7 x 102 g Fe 31

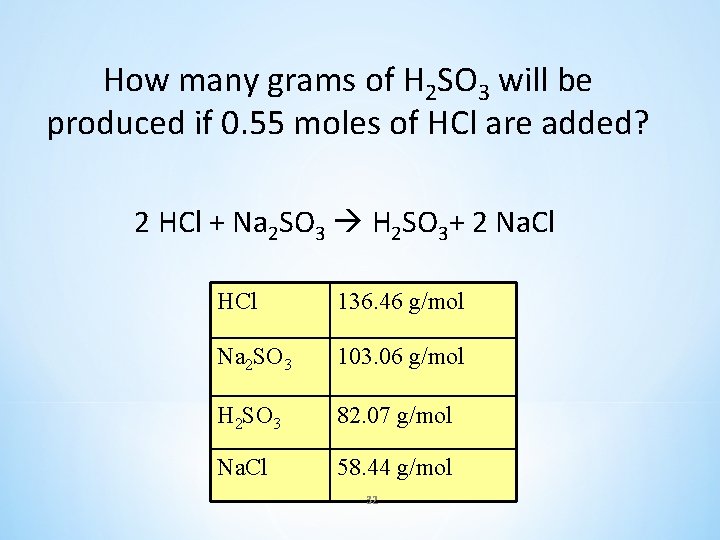
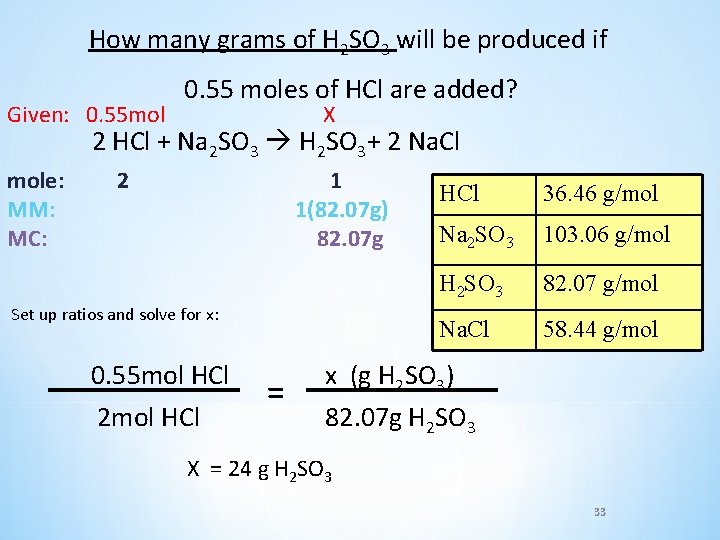
How many grams of H 2 SO 3 will be produced if 0. 55 moles of HCl are added? 2 HCl + Na 2 SO 3 H 2 SO 3+ 2 Na. Cl HCl 136. 46 g/mol Na 2 SO 3 103. 06 g/mol H 2 SO 3 82. 07 g/mol Na. Cl 58. 44 g/mol 32

How many grams of H 2 SO 3 will be produced if Given: 0. 55 mol 0. 55 moles of HCl are added? X 2 HCl + Na 2 SO 3 H 2 SO 3+ 2 Na. Cl mole: MM: MC: 2 1 1(82. 07 g) 82. 07 g Set up ratios and solve for x: 0. 55 mol HCl 2 mol HCl = HCl 36. 46 g/mol Na 2 SO 3 103. 06 g/mol H 2 SO 3 82. 07 g/mol Na. Cl 58. 44 g/mol x (g H 2 SO 3) 82. 07 g H 2 SO 3 X = 24 g H 2 SO 3 33

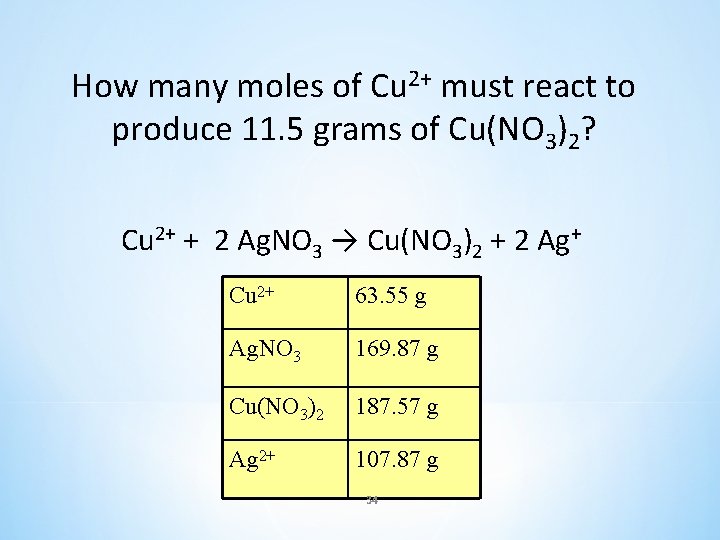
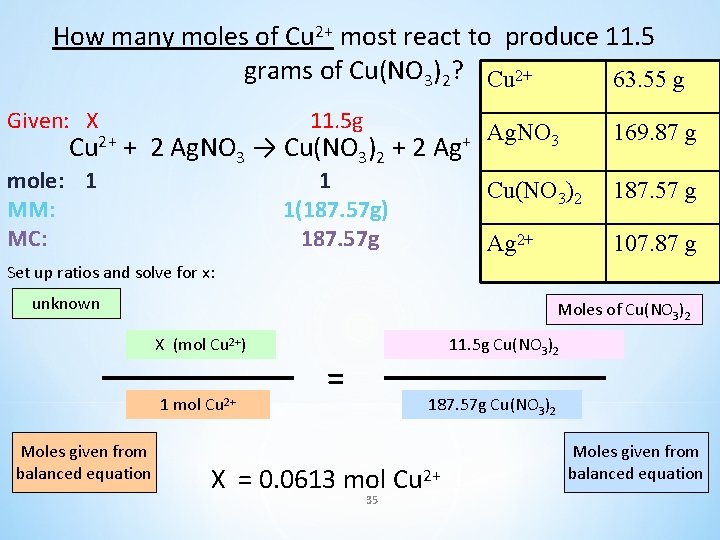
How many moles of Cu 2+ must react to produce 11. 5 grams of Cu(NO 3)2? Cu 2+ + 2 Ag. NO 3 → Cu(NO 3)2 + 2 Ag+ Cu 2+ 63. 55 g Ag. NO 3 169. 87 g Cu(NO 3)2 187. 57 g Ag 2+ 107. 87 g 34

How many moles of Cu 2+ most react to produce 11. 5 grams of Cu(NO 3)2? Cu 2+ 63. 55 g Given: X Cu 2+ mole: 1 MM: MC: 11. 5 g + 2 Ag. NO 3 → Cu(NO 3)2 + 2 Ag+ 1 1(187. 57 g) 187. 57 g Ag. NO 3 169. 87 g Cu(NO 3)2 187. 57 g Ag 2+ 107. 87 g Set up ratios and solve for x: unknown Moles of Cu(NO 3)2 X (mol Cu 2+) 1 mol Cu 2+ Moles given from balanced equation 11. 5 g Cu(NO 3)2 = 187. 57 g Cu(NO 3)2 X = 0. 0613 mol Cu 2+ 35 Moles given from balanced equation

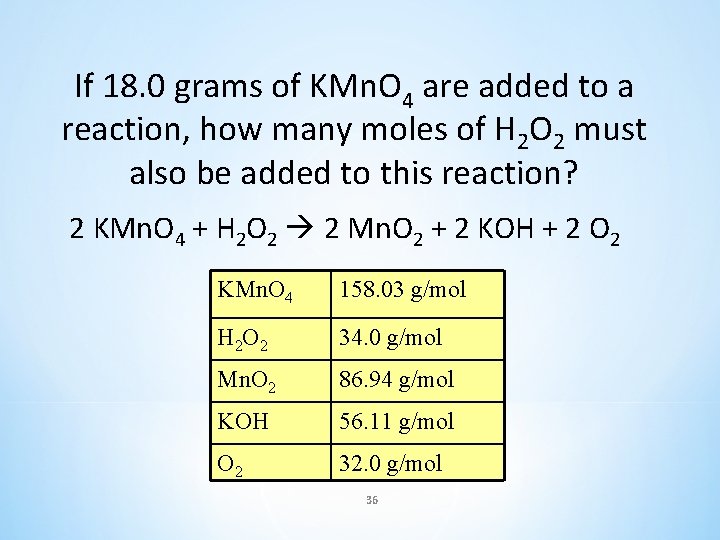
If 18. 0 grams of KMn. O 4 are added to a reaction, how many moles of H 2 O 2 must also be added to this reaction? 2 KMn. O 4 + H 2 O 2 2 Mn. O 2 + 2 KOH + 2 O 2 KMn. O 4 158. 03 g/mol H 2 O 2 34. 0 g/mol Mn. O 2 86. 94 g/mol KOH 56. 11 g/mol O 2 32. 0 g/mol 36

KMn. O 4 If 18. 0 grams of KMn. O 4 are reacted, how many moles of H 2 O 2 must also be added to this Mn. O 2 reaction? Given: 18. 0 g X 158. 03 g/mol 34. 0 g/mol 86. 94 g/mol KOH 56. 11 g/mol O 2 32. 0 g/mol 2 KMn. O 4 + H 2 O 2 2 Mn. O 2 + 2 KOH + 2 O 2 mole: 2 MM: 2(158. 03 g) MC: 316. 06 g 1 18. 0 g KMn. O 4 316. 06 g KMn. O 4 = X (mol H 2 O 2) 1 mol H 2 O 2 37 H O X = 0. 0570 mol 2 2

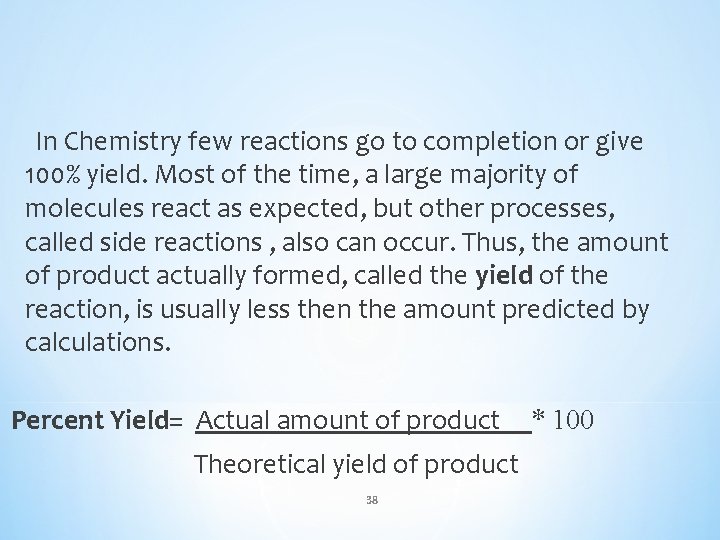
In Chemistry few reactions go to completion or give 100% yield. Most of the time, a large majority of molecules react as expected, but other processes, called side reactions , also can occur. Thus, the amount of product actually formed, called the yield of the reaction, is usually less then the amount predicted by calculations. Percent Yield= Actual amount of product Theoretical yield of product 38 * 100

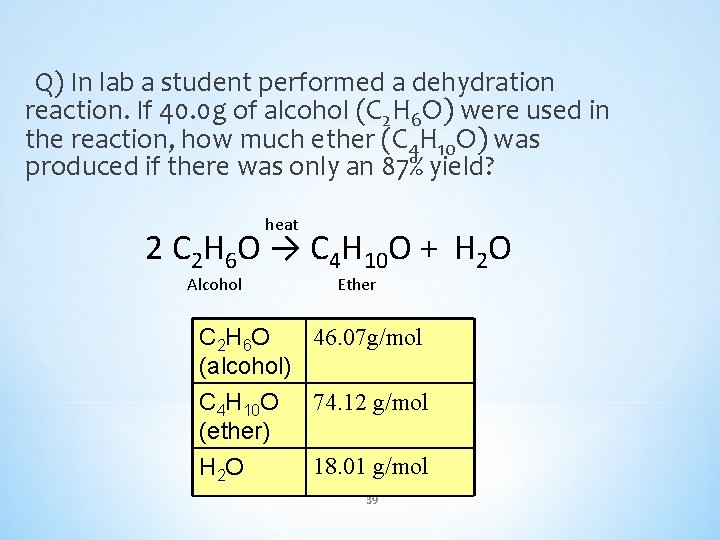
Q) In lab a student performed a dehydration reaction. If 40. 0 g of alcohol (C 2 H 6 O) were used in the reaction, how much ether (C 4 H 10 O) was produced if there was only an 87% yield? heat 2 C 2 H 6 O → C 4 H 10 O + H 2 O Alcohol Ether 46. 07 g/mol C 2 H 6 O (alcohol) C 4 H 10 O 74. 12 g/mol (ether) 18. 01 g/mol H 2 O 39

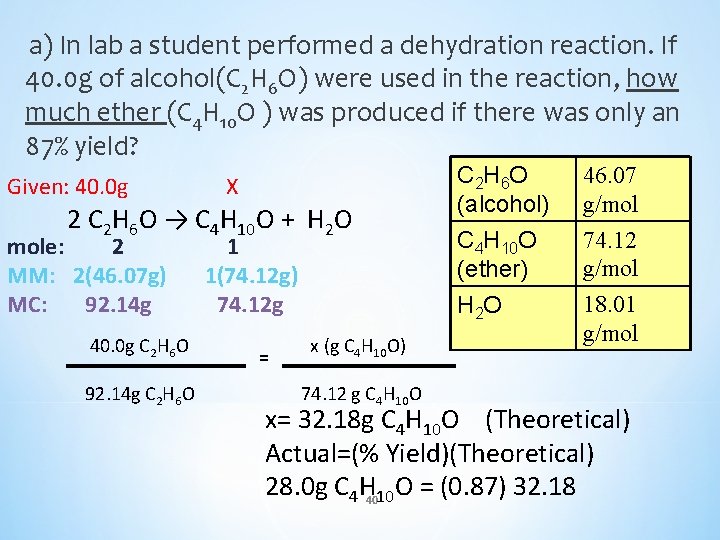
a) In lab a student performed a dehydration reaction. If 40. 0 g of alcohol(C 2 H 6 O) were used in the reaction, how much ether (C 4 H 10 O ) was produced if there was only an 87% yield? Given: 40. 0 g X 2 C 2 H 6 O → C 4 H 10 O + H 2 O mole: 2 MM: 2(46. 07 g) MC: 92. 14 g 40. 0 g C 2 H 6 O 92. 14 g C 2 H 6 O 1 1(74. 12 g) 74. 12 g = x (g C 4 H 10 O) 74. 12 g C 4 H 10 O C 2 H 6 O (alcohol) C 4 H 10 O (ether) H 2 O 46. 07 g/mol 74. 12 g/mol 18. 01 g/mol x= 32. 18 g C 4 H 10 O (Theoretical) Actual=(% Yield)(Theoretical) 28. 0 g C 4 H 4010 O = (0. 87) 32. 18

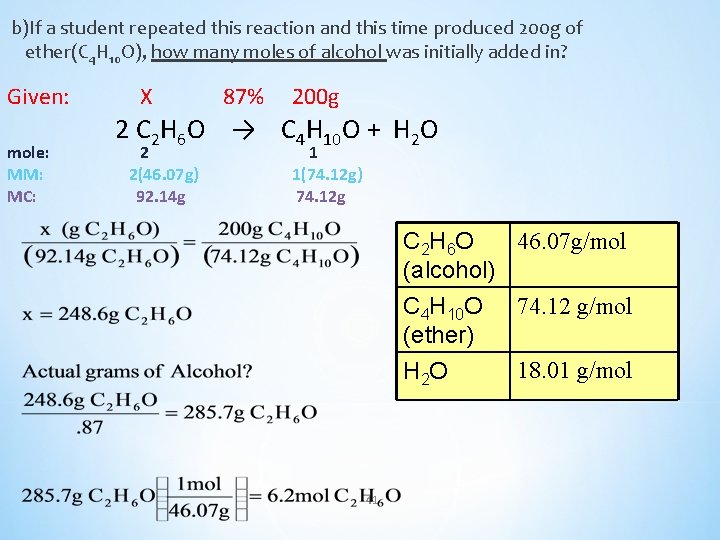
b)If a student repeated this reaction and this time produced 200 g of ether(C 4 H 10 O), how many moles of alcohol was initially added in? Given: mole: MM: MC: X 87% 200 g 2 C 2 H 6 O → C 4 H 10 O + H 2 O 2 2(46. 07 g) 92. 14 g 1 1(74. 12 g) 74. 12 g 46. 07 g/mol C 2 H 6 O (alcohol) C 4 H 10 O 74. 12 g/mol (ether) 18. 01 g/mol H 2 O 41

In a reaction, if one reagent is completely used up before the other, then that reagent is the Limiting Reagent (LR). All other reagents are labeled Excess Reagents (ER). 42
![Q Urea NH 22 CO is produced by reacting ammonia with carbon dioxide For Q) Urea [(NH 2)2 CO] is produced by reacting ammonia with carbon dioxide. For](https://slidetodoc.com/presentation_image_h2/e683762aaf453cf477034d7867156809/image-43.jpg)
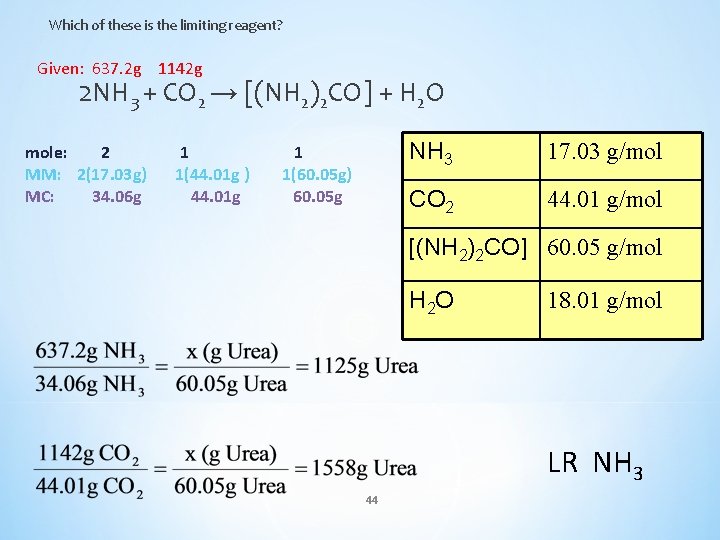
Q) Urea [(NH 2)2 CO] is produced by reacting ammonia with carbon dioxide. For example 637. 2 g of NH 3 can react with 1142 g CO 2 but wait, which is the LR and which is the ER? 2 NH 3 + CO 2 → [(NH 2)2 CO] + H 2 O NH 3 17. 03 g/mol CO 2 44. 01 g/mol [(NH 2)2 CO] 60. 05 g/mol H 2 O 18. 01 g/mol 43

Which of these is the limiting reagent? Given: 637. 2 g 1142 g 2 NH 3 + CO 2 → [(NH 2)2 CO] + H 2 O mole: 2 MM: 2(17. 03 g) MC: 34. 06 g 1 1(44. 01 g ) 44. 01 g 1 1(60. 05 g) 60. 05 g NH 3 17. 03 g/mol CO 2 44. 01 g/mol [(NH 2)2 CO] 60. 05 g/mol H 2 O 18. 01 g/mol LR NH 3 44

How many grams of CO 2 will be left over in this reaction (Remember NH 3 is the LR)? 45

Titration: Procedure for determining the concentration (think Molarity M) of an unknown in an Acid/Base Neutralization or Redox reaction. 46

Q) Calculate the Molarity of a solution containing 0. 2 mol of sodium hydroxide dissolved in 500 m. L of water. 47

Q) Calculate the Molarity of a solution containing 0. 2 mol of sodium hydroxide dissolved in 500 m. L of water. M= mole solute Volume solvent Given: 0. 2 mol Na. OH in 500 m. L H 2 O 48

Q) What volume of 0. 250 M Sulfuric Acid is needed to react with 50. 0 m. L of a 0. 100 M Sodium Hydroxide solution? H 2 SO 4 + 2 Na. OH → Na 2 SO 4 + 2 H 2 O 49

* Q) What volume of 0. 250 M Sulfuric Acid is needed to react with 50. 0 m. L of a 0. 100 M Sodium Hydroxide solution? a. How many moles are in 50 m. L of 0. 100 M Na. OH solution? M= mole Volume 50

Given: X 0. 005 mol H 2 SO 4 + 2 Na. OH → Na 2 SO 4 + 2 H 2 O mole: 1 2 Q) What volume of 0. 250 M Sulfuric Acid is needed to react with 50. 0 m. L of a 0. 100 M Sodium Hydroxide solution? b. How many moles of H 2 SO 4 can neutralize 0. 005 moles of Na. OH? 51

c. What is the volume of 0. 250 M H 2 SO 4 needed for this titration (Remember we found 0. 0025 mol H 2 SO 4 are needed in the last step)? M= mole Volume 52

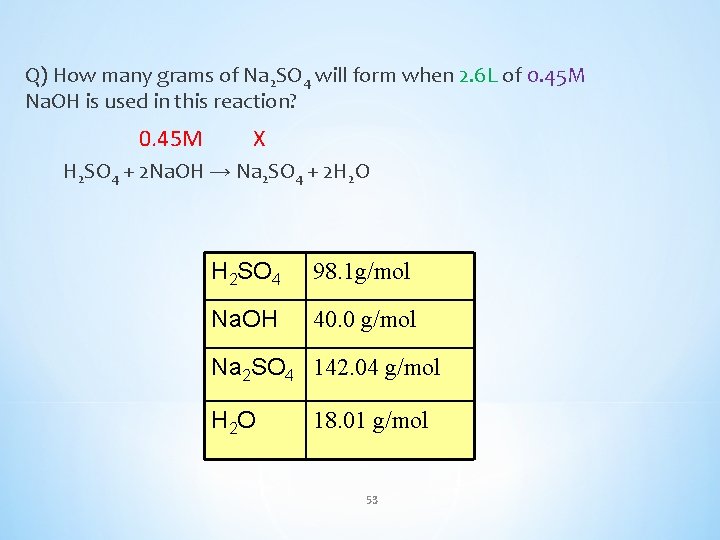
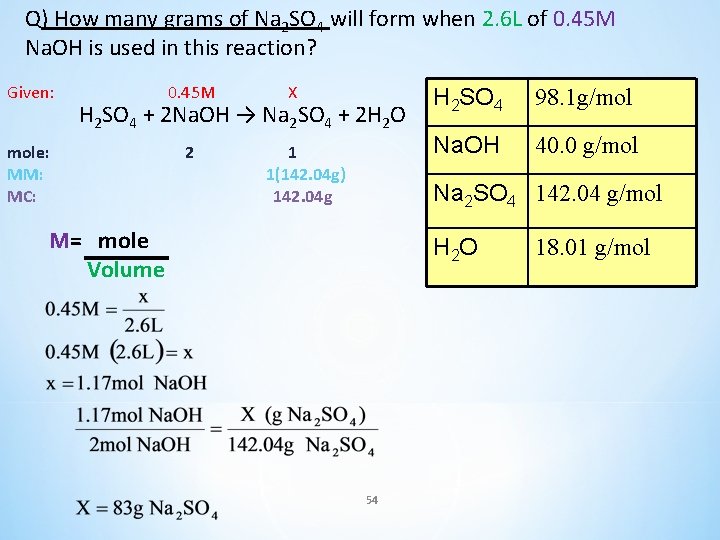
Q) How many grams of Na 2 SO 4 will form when 2. 6 L of 0. 45 M Na. OH is used in this reaction? 0. 45 M X H 2 SO 4 + 2 Na. OH → Na 2 SO 4 + 2 H 2 O H 2 SO 4 98. 1 g/mol Na. OH 40. 0 g/mol Na 2 SO 4 142. 04 g/mol H 2 O 18. 01 g/mol 53

Q) How many grams of Na 2 SO 4 will form when 2. 6 L of 0. 45 M Na. OH is used in this reaction? Given: 0. 45 M X H 2 SO 4 + 2 Na. OH → Na 2 SO 4 + 2 H 2 O mole: MM: MC: 2 1 1(142. 04 g) 142. 04 g H 2 SO 4 98. 1 g/mol Na. OH 40. 0 g/mol Na 2 SO 4 142. 04 g/mol M= mole Volume H 2 O 54 18. 01 g/mol

Dilutions Moles solute(constant)=molarity*Volume Mol solute= M Mi. Vi=Mf. Vf 55 * V

Q) A 200 m. L sample of 6. 7 M H 2 SO 4 is diluted with 800 m. L of H 2 O. What is the new molarity of the solution? M i V i = M f. V f M i. Vi = M f Vf 56

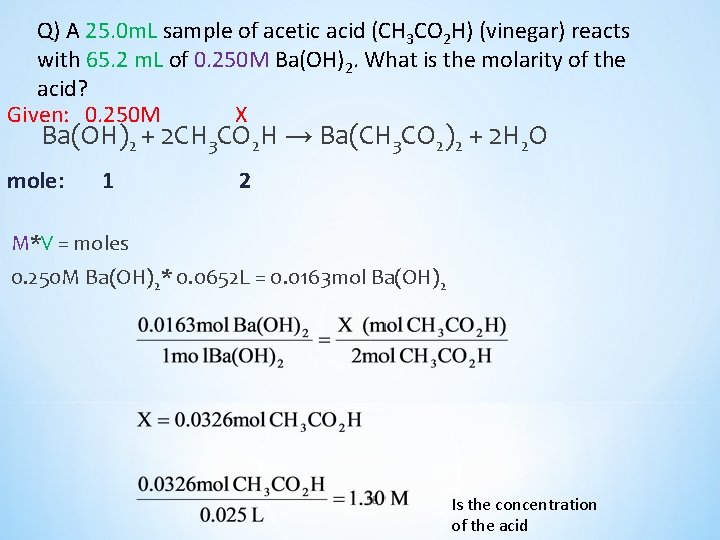
Q) A 25. 0 m. L sample of acetic acid (CH 3 CO 2 H) (vinegar) reacts with 65. 2 m. L of 0. 250 M Ba(OH)2. What is the molarity of the acid? Ba(OH)2 + 2 CH 3 CO 2 H → Ba(CH 3 CO 2)2 + 2 H 2 O 0. 250 M X 57

Q) A 25. 0 m. L sample of acetic acid (CH 3 CO 2 H) (vinegar) reacts with 65. 2 m. L of 0. 250 M Ba(OH)2. What is the molarity of the acid? Given: 0. 250 M X Ba(OH)2 + 2 CH 3 CO 2 H → Ba(CH 3 CO 2)2 + 2 H 2 O mole: 1 2 M*V = moles 0. 250 M Ba(OH)2* 0. 0652 L = 0. 0163 mol Ba(OH)2 58 Is the concentration of the acid

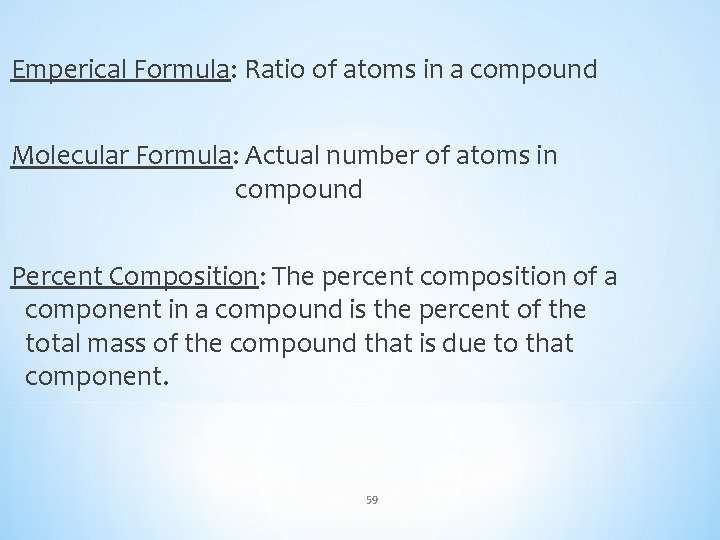
Emperical Formula: Ratio of atoms in a compound Molecular Formula: Actual number of atoms in compound Percent Composition: The percent composition of a component in a compound is the percent of the total mass of the compound that is due to that component. 59

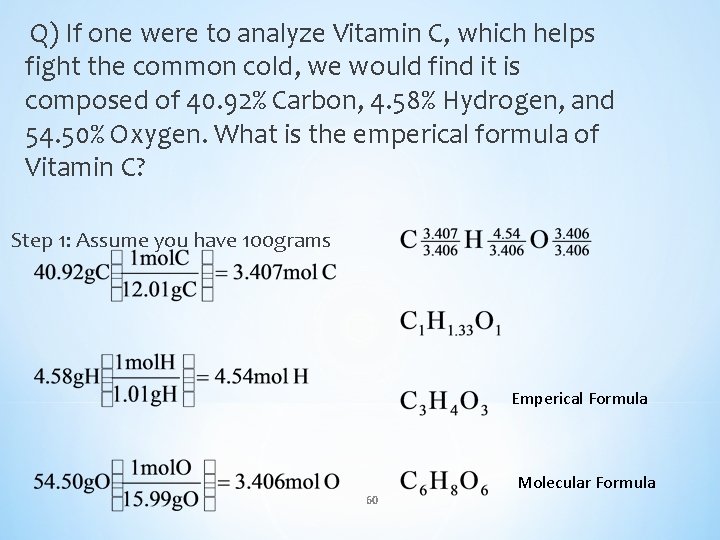
Q) If one were to analyze Vitamin C, which helps fight the common cold, we would find it is composed of 40. 92% Carbon, 4. 58% Hydrogen, and 54. 50% Oxygen. What is the emperical formula of Vitamin C? Step 1: Assume you have 100 grams Emperical Formula 60 Molecular Formula

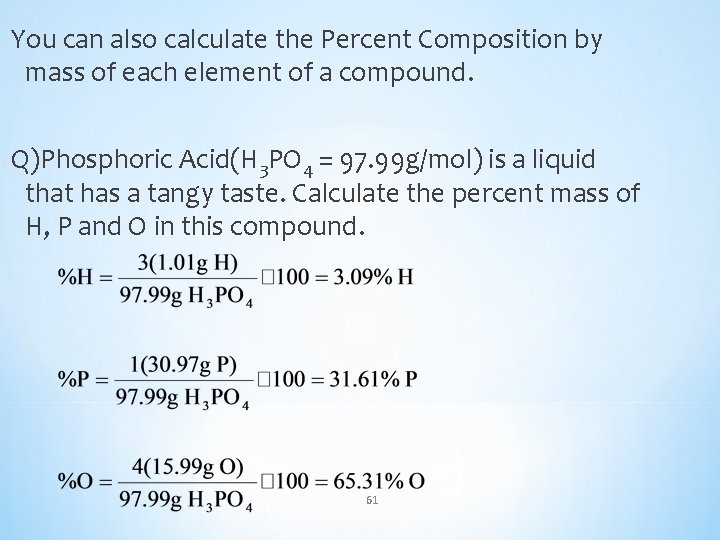
You can also calculate the Percent Composition by mass of each element of a compound. Q)Phosphoric Acid(H 3 PO 4 = 97. 99 g/mol) is a liquid that has a tangy taste. Calculate the percent mass of H, P and O in this compound. 61

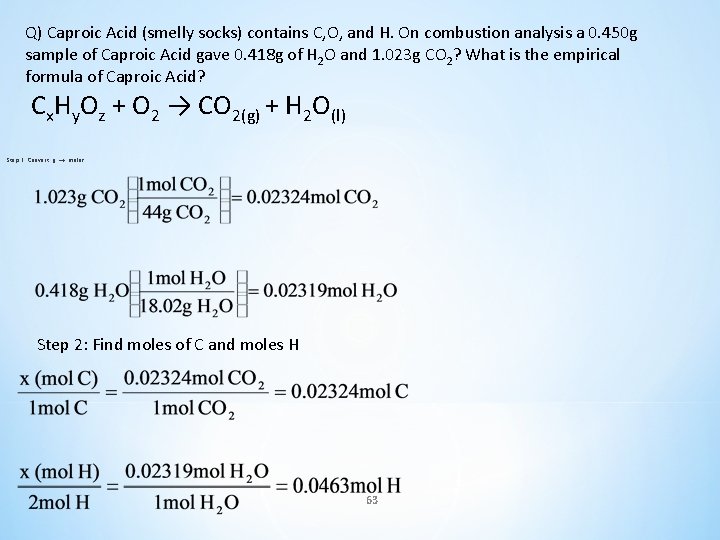
Q) Caproic Acid (smelly socks) contains C, O, and H. On combustion analysis a 0. 450 g sample of Caproic Acid gave 0. 418 g of H 2 O and 1. 023 g CO 2? What is the empirical formula of Caproic Acid? 0. 450 g 1. 023 g 0. 418 g Cx. Hy. Oz + O 2 → CO 2(g) + H 2 O(l) 62

Q) Caproic Acid (smelly socks) contains C, O, and H. On combustion analysis a 0. 450 g sample of Caproic Acid gave 0. 418 g of H 2 O and 1. 023 g CO 2? What is the empirical formula of Caproic Acid? Cx. Hy. Oz + O 2 → CO 2(g) + H 2 O(l) Step 1: Convert g → moles Step 2: Find moles of C and moles H 63

Step 3) Convert to g of C and H Step 4) Compare Values 64

Additional Problems 65
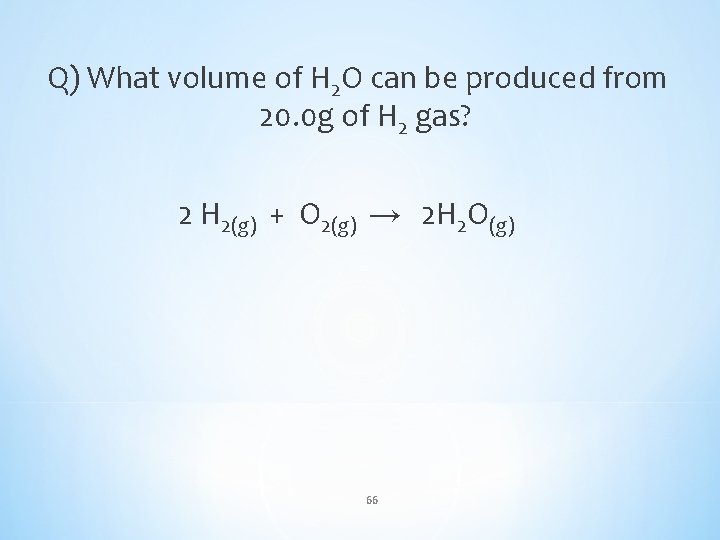
Q) What volume of H 2 O can be produced from 20. 0 g of H 2 gas? 2 H 2(g) + O 2(g) → 2 H 2 O(g) 66

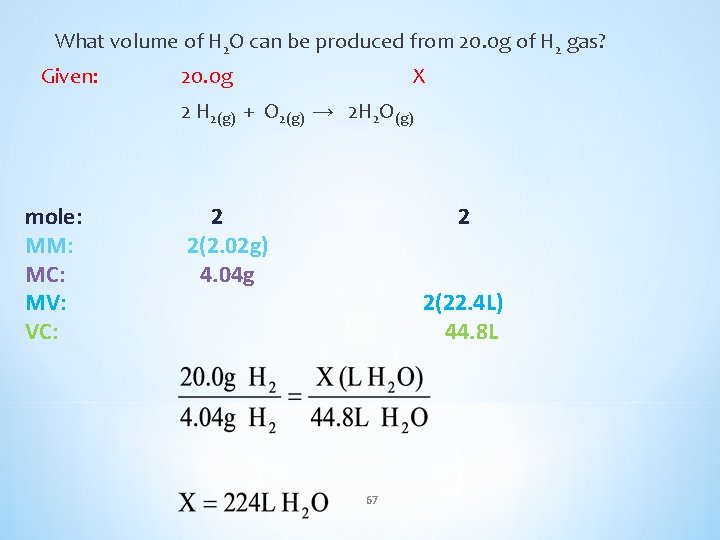
What volume of H 2 O can be produced from 20. 0 g of H 2 gas? Given: 20. 0 g X 2 H 2(g) + O 2(g) → 2 H 2 O(g) mole: MM: MC: MV: VC: 2 2(2. 02 g) 4. 04 g 2 2(22. 4 L) 44. 8 L 67

Q) The specific heat of iron metal is 0. 450 J/g-K. How many J of heat are necessary to raise the temperature of a 1. 05 -kg block of iron from 25. 0ºC to 88. 5ºC ? 68

The specific heat of iron metal is 0. 450 J/g-K. How many J of heat are necessary to raise the temperature of a 1. 05 -kg block of iron from 25. 0ºC to 88. 5ºC ? Q= mc ∆T c= 0. 450 J/g ºC mass= 1. 05 kg ∆T=(88. 5 -25. 0) ºC = 63. 5ºC Q= ? 69
 What is sum of roots in quadratic equation
What is sum of roots in quadratic equation What is the perfect square of 300
What is the perfect square of 300 What's a perfect square
What's a perfect square Lesson 3 existence and uniqueness
Lesson 3 existence and uniqueness Economic roots of american imperialism
Economic roots of american imperialism Chonp
Chonp Stoicheion meaning
Stoicheion meaning Engertian
Engertian Stoicheion
Stoicheion Stoicheion
Stoicheion Stoicheion meaning
Stoicheion meaning An element of art that is derived from reflected light
An element of art that is derived from reflected light Greek words in physics
Greek words in physics Where does the word polygon come from
Where does the word polygon come from Technology came from the two greek word, *
Technology came from the two greek word, * Photography comes from the greek words photos
Photography comes from the greek words photos Geometry meaning in greek
Geometry meaning in greek Trigonometry is derived from greek words
Trigonometry is derived from greek words Ichthy root word
Ichthy root word Puls latin root
Puls latin root Vocabulary from latin and greek roots unit 11
Vocabulary from latin and greek roots unit 11 Psychology greek roots
Psychology greek roots Identify the meaning of the root sta/sti.
Identify the meaning of the root sta/sti. Vocabulary from latin and greek roots unit one
Vocabulary from latin and greek roots unit one Root word anthrop
Root word anthrop Vig latin root words
Vig latin root words Latin root temp
Latin root temp Greek and latin roots jeopardy
Greek and latin roots jeopardy Root sta/sti
Root sta/sti Greek and latin roots and affixes unit 1 answer key
Greek and latin roots and affixes unit 1 answer key Latin root fort
Latin root fort Words with liter root
Words with liter root Words with the greek root therm
Words with the greek root therm Greek or latin root/affix for hosp
Greek or latin root/affix for hosp Muscular system latin
Muscular system latin All greek roots
All greek roots Words with root spec
Words with root spec Tele root word membean
Tele root word membean Vocabulary from latin and greek roots unit 8
Vocabulary from latin and greek roots unit 8 Astronomy greek roots
Astronomy greek roots Greek root vis
Greek root vis Legare latin
Legare latin Meter latin root meaning
Meter latin root meaning Greek root for fear
Greek root for fear Vinc root word examples
Vinc root word examples Words with greek root tele
Words with greek root tele Using greek and latin roots and affixes
Using greek and latin roots and affixes Grad gress root word
Grad gress root word Distinguish between a signal element and a data element.
Distinguish between a signal element and a data element. Signal element vs data element
Signal element vs data element What is the greek miracle in greek mythology
What is the greek miracle in greek mythology Diễn thế sinh thái là
Diễn thế sinh thái là Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Phép trừ bù
Phép trừ bù Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Lời thề hippocrates
Lời thề hippocrates đại từ thay thế
đại từ thay thế Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Công thức tiính động năng
Công thức tiính động năng Các môn thể thao bắt đầu bằng tiếng đua
Các môn thể thao bắt đầu bằng tiếng đua Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ Phản ứng thế ankan
Phản ứng thế ankan Gấu đi như thế nào
Gấu đi như thế nào Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan điện thế nghỉ
điện thế nghỉ