Stoichiometry From the Greek words stoicheion meaning element




- Slides: 16

Stoichiometry From the Greek words “stoicheion” meaning “element” and “metron” meaning “measure”

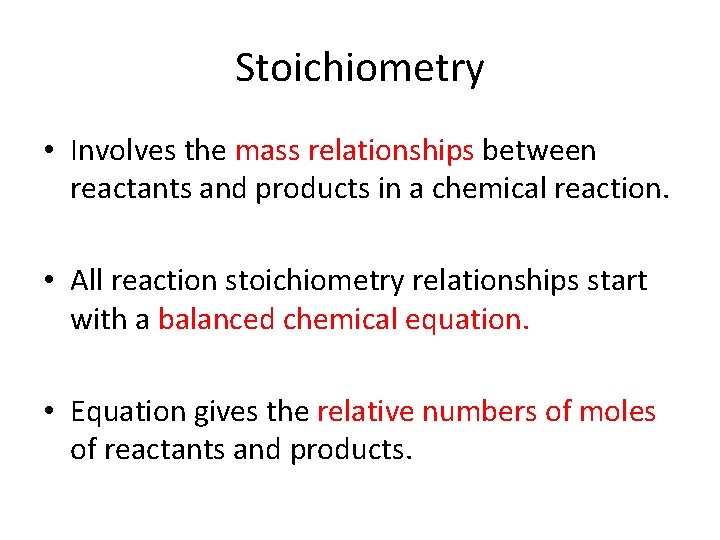
Stoichiometry • Involves the mass relationships between reactants and products in a chemical reaction. • All reaction stoichiometry relationships start with a balanced chemical equation. • Equation gives the relative numbers of moles of reactants and products.

Stoichiometry • Solve problems using RATIOS from the balanced equation.

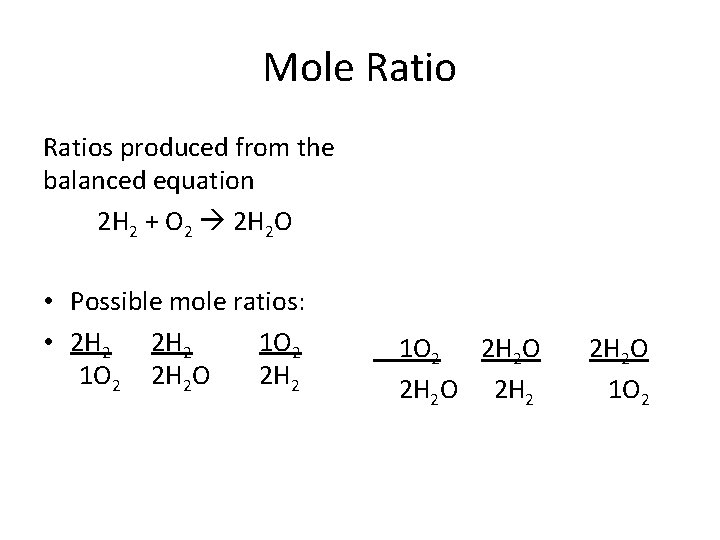
Mole Ratios produced from the balanced equation 2 H 2 + O 2 2 H 2 O • Possible mole ratios: • 2 H 2 1 O 2 2 H 2 O 2 H 2 O 1 O 2

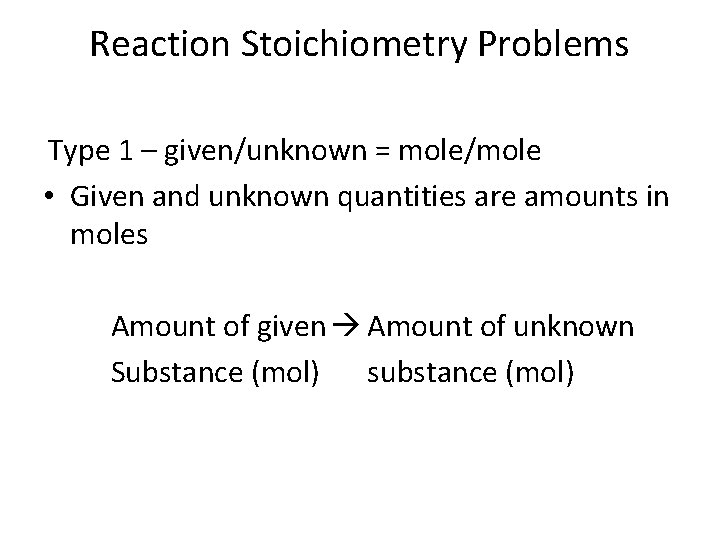
Reaction Stoichiometry Problems Type 1 – given/unknown = mole/mole • Given and unknown quantities are amounts in moles Amount of given Amount of unknown Substance (mol) substance (mol)

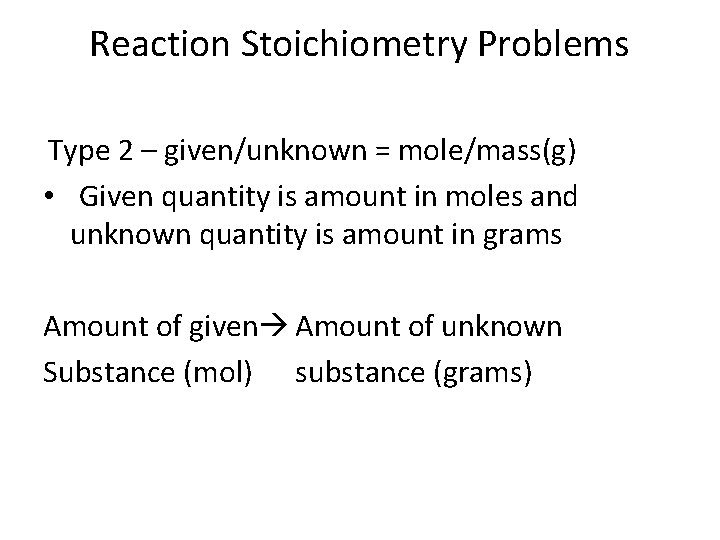
Reaction Stoichiometry Problems Type 2 – given/unknown = mole/mass(g) • Given quantity is amount in moles and unknown quantity is amount in grams Amount of given Amount of unknown Substance (mol) substance (grams)

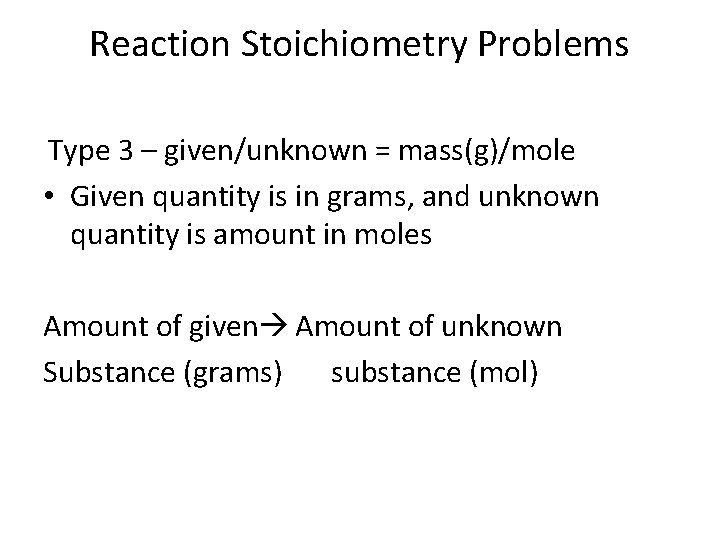
Reaction Stoichiometry Problems Type 3 – given/unknown = mass(g)/mole • Given quantity is in grams, and unknown quantity is amount in moles Amount of given Amount of unknown Substance (grams) substance (mol)

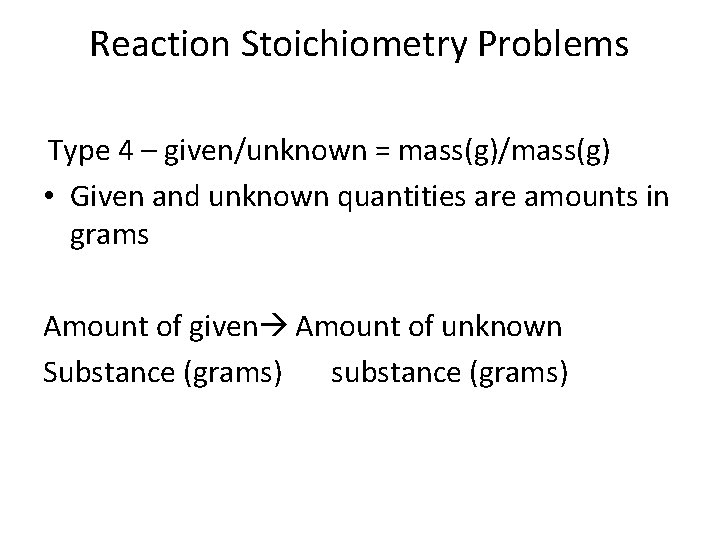
Reaction Stoichiometry Problems Type 4 – given/unknown = mass(g)/mass(g) • Given and unknown quantities are amounts in grams Amount of given Amount of unknown Substance (grams) substance (grams)

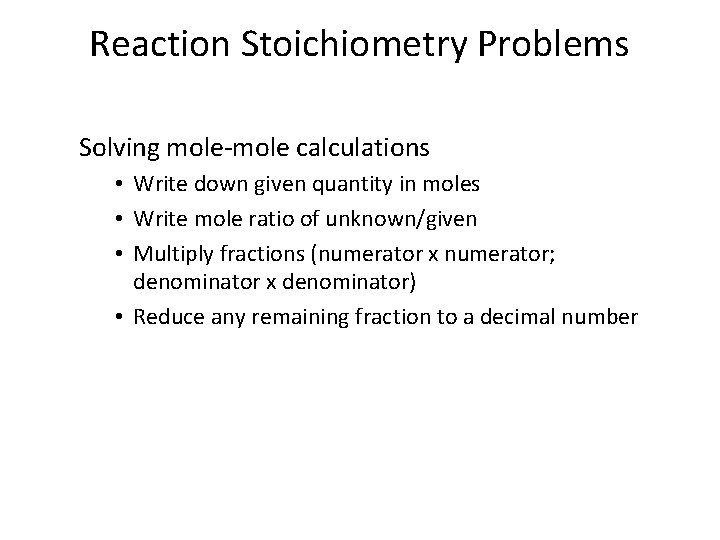
Reaction Stoichiometry Problems Solving mole-mole calculations • Write down given quantity in moles • Write mole ratio of unknown/given • Multiply fractions (numerator x numerator; denominator x denominator) • Reduce any remaining fraction to a decimal number

Reaction Stoichiometry Problems Solving mole to mass calculations • Write down given quantity in moles • Multiply by mole ratio of unknown/given • Multiply fractions (numerator x numerator; denominator x denominator) • Reduce any remaining fraction to a decimal number • Multiply by molar mass of unknown.

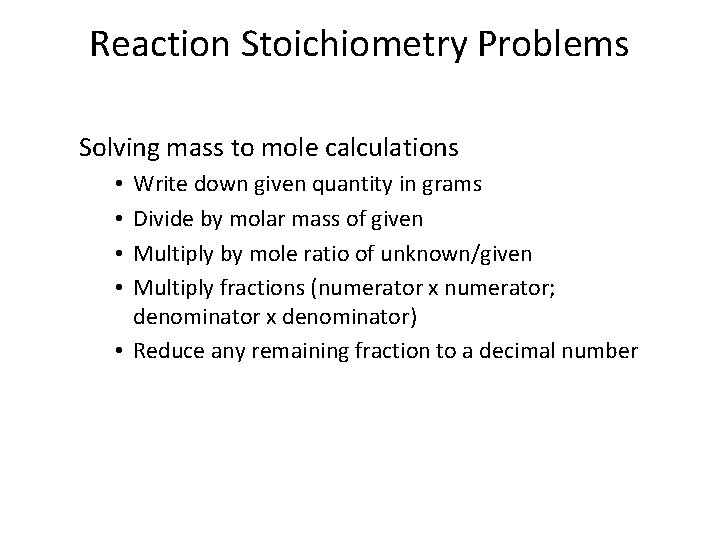
Reaction Stoichiometry Problems Solving mass to mole calculations Write down given quantity in grams Divide by molar mass of given Multiply by mole ratio of unknown/given Multiply fractions (numerator x numerator; denominator x denominator) • Reduce any remaining fraction to a decimal number • •

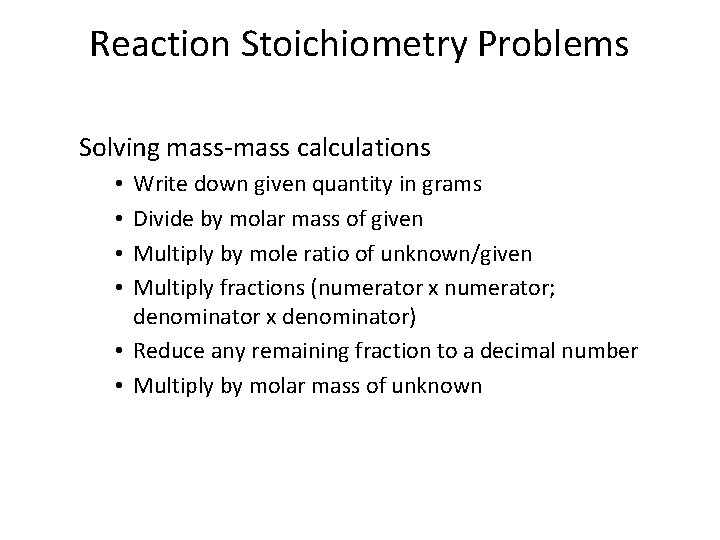
Reaction Stoichiometry Problems Solving mass-mass calculations Write down given quantity in grams Divide by molar mass of given Multiply by mole ratio of unknown/given Multiply fractions (numerator x numerator; denominator x denominator) • Reduce any remaining fraction to a decimal number • Multiply by molar mass of unknown • •

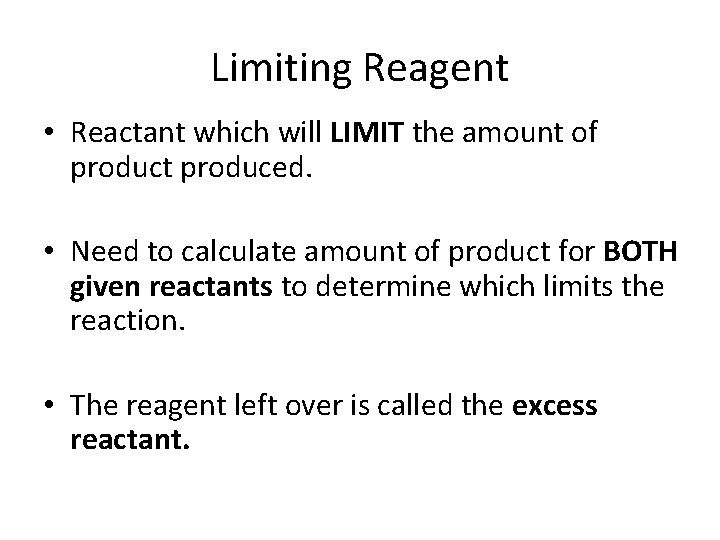
Limiting Reagent • Reactant which will LIMIT the amount of product produced. • Need to calculate amount of product for BOTH given reactants to determine which limits the reaction. • The reagent left over is called the excess reactant.

Theoretical Yield Amount of product made under IDEAL conditions. The most possible product you can make. Almost never happens. This is the number you calculate using stoichiometry.

Actual Yield • Amount of product made during the actual reaction in the laboratory.

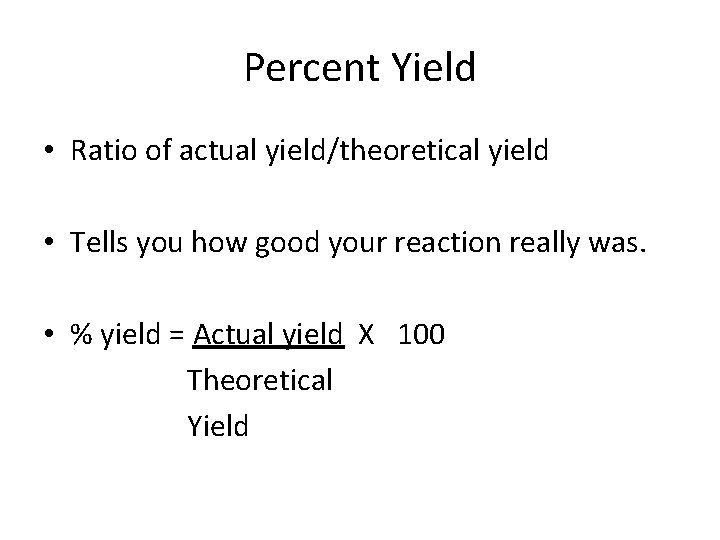
Percent Yield • Ratio of actual yield/theoretical yield • Tells you how good your reaction really was. • % yield = Actual yield X 100 Theoretical Yield
 Stoicheion elements
Stoicheion elements What does stoichiometry mean in greek
What does stoichiometry mean in greek Stoicheion
Stoicheion Stoicheion
Stoicheion Stoicheion
Stoicheion Polygon comes from the greek word
Polygon comes from the greek word Geometry greek meaning
Geometry greek meaning 5 a day language review week 14
5 a day language review week 14 Difference between signal element and data element
Difference between signal element and data element Signal element vs data element
Signal element vs data element What is the greek miracle in greek mythology
What is the greek miracle in greek mythology Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Slidetodoc
Slidetodoc Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Chụp phim tư thế worms-breton
Chụp phim tư thế worms-breton