The Gas Laws Properties of Gases Assumed to

































- Slides: 35

The Gas Laws

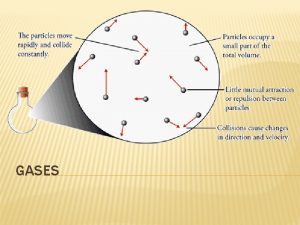
Properties of Gases Assumed to be hard spherical particles (atoms or molecules) Extremely small compared to the distances between them which makes them highly compressible (flatten of squeeze together by force) Low density Have no attractive or repulsive forces between particles ◦ Free to move inside their containers ◦ No definite shape (expand until it takes the shape of its container) ◦ Completely fill a container and exert pressure in all directions (no definite volume) Fluid (meaning the “flow” the molecules are in constant motion and can move past each other and have no definite shape) Particles travel in a straight path and only deviate from the path when it collides with another gas particle. Collisions between gas particles are considered to be “elastic” (meaning the total amount of kinetic energy is transferred without loss from one particle to another)

Kinetic Molecular Theory (KMT) Kinetic Molecular Theory of gases attempts to explain the properties of gases such as pressure, temperature, or volume, by looking at what they are made up of and how they move

Kinetic Molecular Theory (KMT) Kinetic refers to motion The energy an object has because of its motion is called kinetic energy ◦ Example: A ball rolling down a hill has kinetic energy

Kinetic Molecular Theory (KMT) There are three main components to kinetic theory: 1. No energy is gained or lost when gas molecules collide 2. The molecules in a gas are so small they take up no space 3. The molecules are in constant, linear, random

Kinetic Molecular Theory (KMT) How does Kinetic Theory explain Gas Pressure? Gas Pressure results from fast moving gas particles colliding with the sides of a container More Collisions = Higher Pressure

Kinetic Molecular Theory (KMT) How does Temperature relate to Kinetic Theory? Temperature is a measure of the average kinetic energy of the particles in a gas Higher Energy = Higher Temperature

Kinetic Molecular Theory (KMT) Through KMT, several Laws were developed to help calculate the changes in pressure, temperature, and volume of gases. There are 6 Basic Laws: 1. Boyle’s Law Combined Gas Law 2. Charles’ Law 3. Gay-Lussac’s Law 4. Avogadro’s Law 5. Ideal Gas Law 6. Dalton’s Law

Units used to describe gas samples: Volume Temperature Pressure Liter (L) Milliliter (m. L) Kelvin ONLY 1000 m. L = 1 L K = ºC + 273 Atmosphere (atm) Kilopascale (k. Pa) Torr (torr) mm of mercury (mm Hg) 1 atm = 101. 3 k. Pa 1 atm = 760 mm Hg 1 atm = 760 torr 1 atm = 14. 7 psi Standard Temperature and Pressure (STP) Standard Temperature = 273 K Standard Pressure = 1 atm

Boyle’s Law – at constant temperature, the volume of the gas increases as the pressure decreases. (and the volume of the gas decreases and the pressure increases). They are inversely related V↑ P↓ V o L u m e P 1 V 1 = P 2 V 2 If you squeeze a gas sample, you make its volume smaller. (L) Pressure (k. Pa)

Now. . . a container where the volume can change (syringe) Moveable piston ↕ Same temperature Volume is 100 m. L at 25°C Volume is 50 m. L at 25°C In which system is the pressure higher? (Which has the greater number of collisions with the walls and each other? ) Boyle’s Law video example

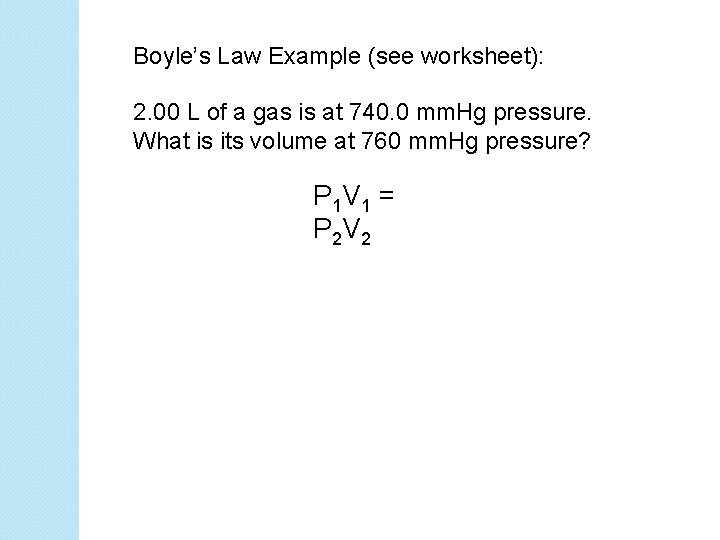
Boyle’s Law Example (see worksheet): 2. 00 L of a gas is at 740. 0 mm. Hg pressure. What is its volume at 760 mm. Hg pressure? P 1 V 1 = P 2 V 2

Charles’ Law – at a constant pressure, the volume of a gas increases as the temperature of the gas increases (and the volume decreases when the temperature decreases). They are directly related. • increase the speed of the particles • the walls of a flexible container expand – think of hot air balloons! V 1 V = 2 T 1 T 2 V o l u m e L Charles’ Law Video Example Temperature (K)

Charles’ Law Example: (on worksheet) 4. 40 L of a gas is collected at 50. 0°C. What will be its volume upon cooling to 25. 0°C? V 1 = V 2 T 1 T 2 Important! First you must convert temperatures from Celsius to Kelvin. Temperature must always be in Kelvin

Gay-Lussac’s Law – at a constant volume, the pressure of a gas increases as the temperature of the gas increases (and the pressure decreases when the temperature decreases). They are directly related. P 1 P 2 = T 1 T 2 Pressure (atm) Gay-Lussac’s Law Video Example Temperature (K)

A B Steel cylinder (2 L) contains 500 molecules of O 2 at 400 K Steel cylinder (2 L) contains 500 molecules of O 2 at 800 K 1. In which system do the O 2 molecules have the highest average B kinetic energy (temperature)? 2. In which system will the particles collide with the container walls with the greatest force and the most often? B 3. In which system is the pressure higher? B

Example: In a rigid container a gas has a pressure of 1. 3 atm at 25°C. What is the pressure of the gas if it is heated to 45°C?

Combined Gas Law A combination of Boyle’s, Charles’, and Gay-Lussac’s Laws P 1 V 1 P 2 V 2 = T 1 T 2 Note that all temperatures must be in Kelvin!

Example: A gas occupies 2. 0 L at 2. 5 atm and 25ºC. What is it’s volume if the temperature is increased to 33ºC and the pressure is decreased to 1. 5 atm? P 1 V 1 P 2 V 2 T 1 T 2

Example: A gas occupies 4. 5 L at 1. 3 atm and 35ºC. What is the final temperature if the final volume of the gas is 3. 2 L with a pressure of 1. 5 atm? P 1 V 1 P 2 V 2 T 1 T 2

Avogadro’s Law – equal volumes of gases at the same temperature and pressure contain equal numbers of molecules. H 2 O 2 CO 2 1 mole of ANY gas takes up a volume of 22. 4 L at STP. This is called Molar Volume

Avogadro’s Law: One mole of ANY gas takes up a volume of 22. 4 L at STP. So how many molecules of any gas are there in 22. 4 L at STP? How many molecules of gas would be in 1. 0 L at STP?

Avogadro’s Law: At STP, 1. 0 L of Helium gas contains the same number of atoms as: A. B. C. D. 2. 0 L of Kr 1. 0 L of Ne 0. 5 L of Rn 1. 5 L of Ar Therefore equal ________ of gas contain equal numbers of _____ or ___________.

You. Tube - Chemistry Music Video 7: Rock Me Avogadro

Ideal Gases • Gases whose behavior can be predicted by the kinetic molecular theory are called ideal, or perfect, gases. No gases are truly ideal because no gas totally obeys all of the gas laws. • An ideal gas is an imaginary gas that is perfect and does follow everything perfectly. • We assume that all gases behave like ideal gases so there is an ideal gas law

Ideal Gas Law PV = n. RT P = pressure in atmospheres (atm) V = volume in Liters (L) n = # of moles T = temperature in Kelvin (K) R =. 08206 L·atm/mol·K

Ideal Gas Law Example: (on worksheet) How many moles of oxygen will occupy a volume of 2. 50 L at 1. 20 atm and 25°C? PV = n. RT

What is STP? STP stands for standard temperature and pressure. Standard temperature is always 273 K. Standard pressure is always 1. 00 atm. Examples using STP: (in notes) At 1. 8 atm of pressure and 30. 0 °C temperature, a gas occupies a volume of 65. 5 m. L. What will be the volume of the same gas at STP? Standard temperature = Standard pressure = Which gas law should we use?

Examples using STP: (on worksheet) How many moles of oxygen will occupy a volume of 2. 50 L at STP? Standard temperature = Standard pressure = Which gas law should we use?

One More Law!! Dalton’s Law of Partial Pressures In a mixture of gases, each gas exerts a certain pressure as if it were alone. The pressure of each one of these gases is called the partial pressure. The total pressure of a mixture of gases is the sum of all of the partial pressures. Ptotal = P 1 + P 2 + P 3 …….

Example: What is the total pressure of a mixture of gases made up of CO 2, and H 2 if the partial pressures are 22. 3 k. Pa, 44. 7 k. Pa, and 112 k. Pa, respectively? Ptotal = P 1 + P 2 + P 3

You. Tube - Myth. Busters - Fun With Gas

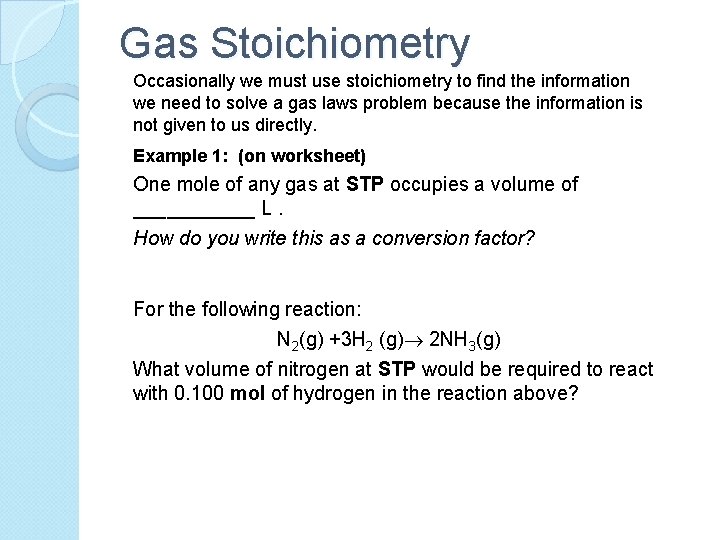
Gas Stoichiometry Occasionally we must use stoichiometry to find the information we need to solve a gas laws problem because the information is not given to us directly. Example 1: (on worksheet) One mole of any gas at STP occupies a volume of ______ L. How do you write this as a conversion factor? For the following reaction: N 2(g) +3 H 2 (g) 2 NH 3(g) What volume of nitrogen at STP would be required to react with 0. 100 mol of hydrogen in the reaction above?

Gas Stoichiometry N 2(g) +3 H 2 (g) 2 NH 3(g) What volume of nitrogen at STP would be required to react with 0. 100 grams of hydrogen in the reaction above?

Gas Stoichiometry Example 2: (on worksheet) Calculate the volume of oxygen gas produced at 1. 00 atm and 25°C when 10. 5 grams of potassium chlorate decomposes. The balanced chemical equation for the reaction is: 3 KCl. O 3 2 KCl + 3 O 2 Which gas law formula must we use to find the volume of the O 2 gas? What must we know before we can use that gas law? How can we calculate it?
 Facts about montesquieu
Facts about montesquieu Buoyancyability
Buoyancyability What are the properties of solids
What are the properties of solids Properties of gas
Properties of gas Four properties of gas
Four properties of gas 5 properties of gases
5 properties of gases Properties of gases
Properties of gases Inert gases properties
Inert gases properties Characteristics of gases
Characteristics of gases Properties of gases
Properties of gases Liperamid
Liperamid Liquid information
Liquid information Properties of gases
Properties of gases Properties of gases
Properties of gases List 2 of the important properties of gases
List 2 of the important properties of gases The imaginary voice assumed by the writer of a poem.
The imaginary voice assumed by the writer of a poem. Who is the assumed speaker of annabel lee
Who is the assumed speaker of annabel lee The imaginary voice assumed by the writer of a poem.
The imaginary voice assumed by the writer of a poem. Assumed mean formula
Assumed mean formula Object deflection shape analysis
Object deflection shape analysis Gas laws crash course
Gas laws crash course Combined gas law direct or indirect
Combined gas law direct or indirect What are the empirical gas laws
What are the empirical gas laws Charles gas law formula
Charles gas law formula All the gas laws
All the gas laws Different gas laws
Different gas laws Charles law
Charles law Conceptual problems examples
Conceptual problems examples Sample problem of boyle's law
Sample problem of boyle's law Chapter 14 the behavior of gases worksheet answer key
Chapter 14 the behavior of gases worksheet answer key 3 gas laws
3 gas laws Different gas laws
Different gas laws Combined gas law worksheet
Combined gas law worksheet Section 13.2 the combined gas law and avogadro's principle
Section 13.2 the combined gas law and avogadro's principle Boyle's law graph
Boyle's law graph Empirical gas laws
Empirical gas laws