Gas Laws NATURE OF GASES Gases are the















- Slides: 39

Gas Laws

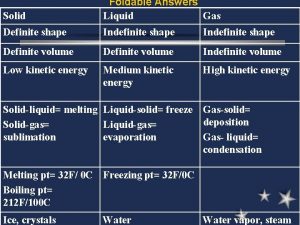
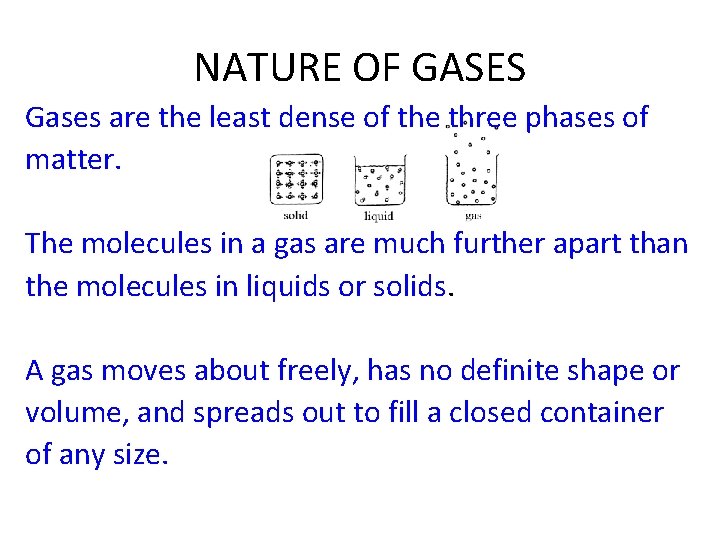
NATURE OF GASES Gases are the least dense of the three phases of matter. The molecules in a gas are much further apart than the molecules in liquids or solids. A gas moves about freely, has no definite shape or volume, and spreads out to fill a closed container of any size.

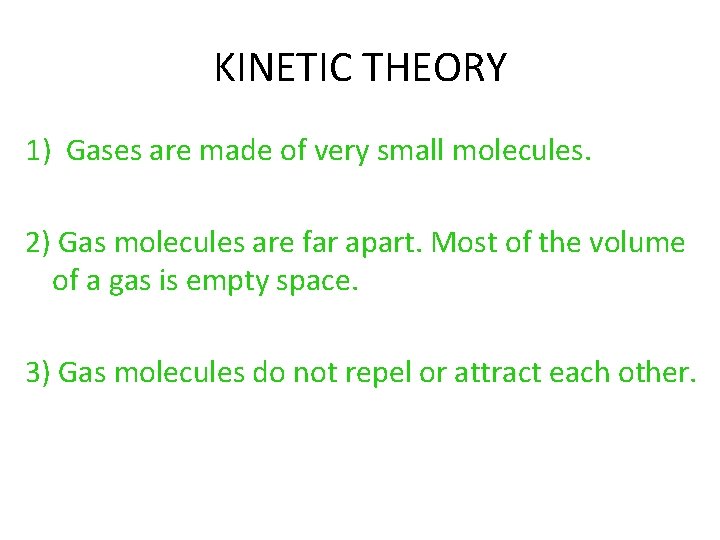
KINETIC THEORY 1) Gases are made of very small molecules. 2) Gas molecules are far apart. Most of the volume of a gas is empty space. 3) Gas molecules do not repel or attract each other.

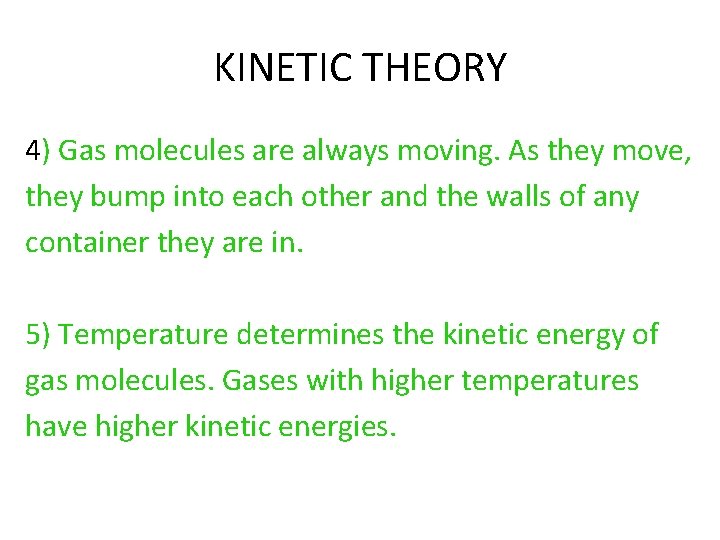
KINETIC THEORY 4) Gas molecules are always moving. As they move, they bump into each other and the walls of any container they are in. 5) Temperature determines the kinetic energy of gas molecules. Gases with higher temperatures have higher kinetic energies.

The volume, pressure and temperature of gases are the conditions measured. VOLUME PRESSURE TEMPERATURE

VOLUME = the total space the gas occupies inside its container. volume is usually measured in units of milliliters (m. L), liters (L) or cubic centimeters (cc) 1 m. L = 1 cc 1000 m. L = 1 L

PRESSURE = is caused by gas molecules moving around and bumping into the sides of the container. Pressure is measured in the units of atmospheres (atm), millimeters of mercury (mm Hg), which are also called torr. They are named for the inventor of the barometer, Torricelli. Standard pressure is 1 atm

PRESSURE CONVERTING PRESSURE 1 atmosphere = 760 mm of Hg = 760 torr 1 atm = 101 kilopascals

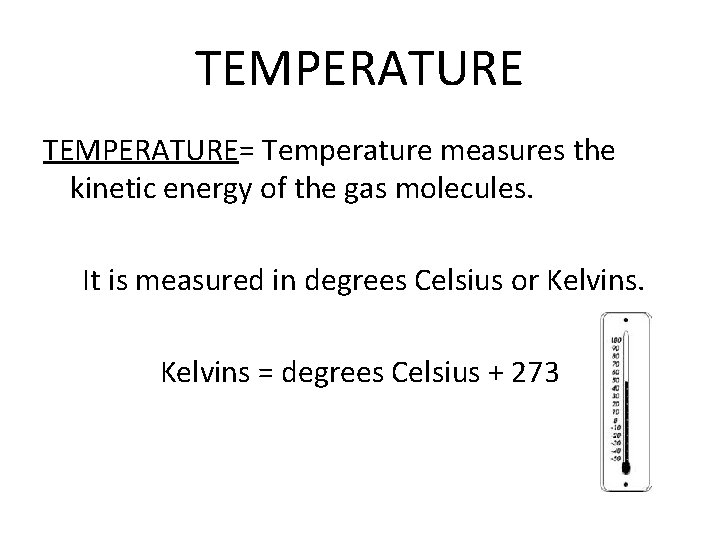
TEMPERATURE= Temperature measures the kinetic energy of the gas molecules. It is measured in degrees Celsius or Kelvins = degrees Celsius + 273

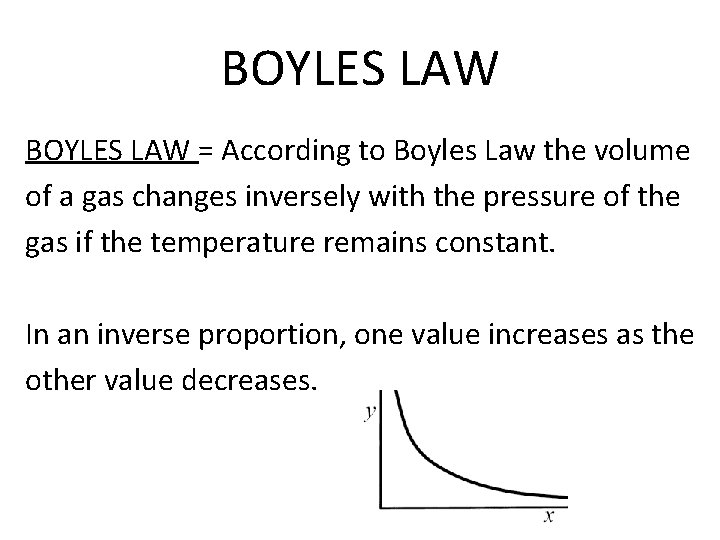
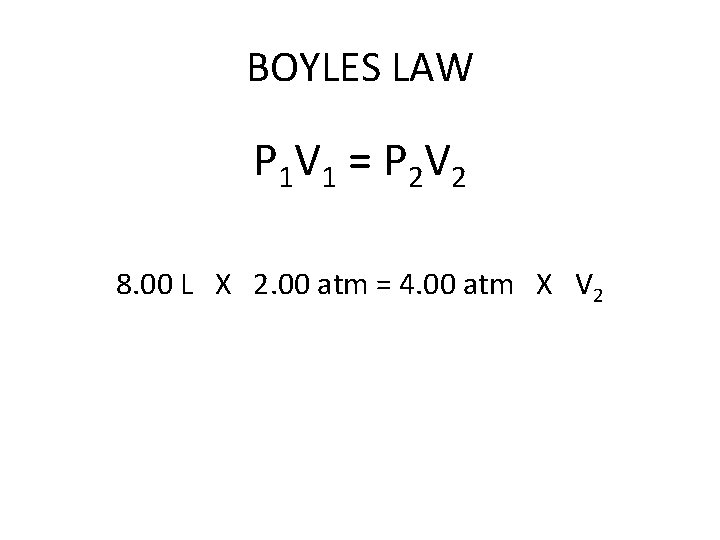
BOYLES LAW = According to Boyles Law the volume of a gas changes inversely with the pressure of the gas if the temperature remains constant. In an inverse proportion, one value increases as the other value decreases.

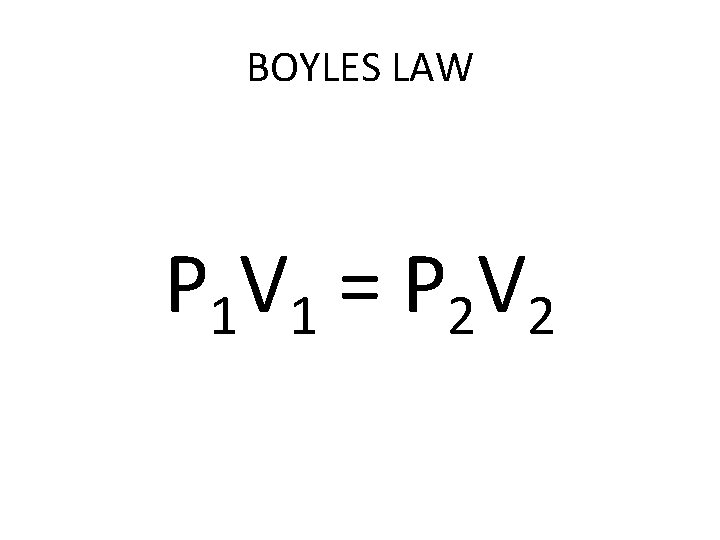
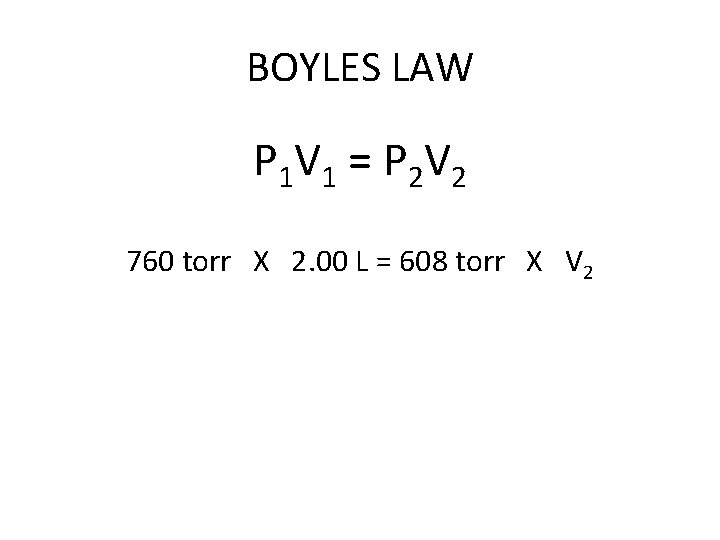
BOYLES LAW P 1 V 1 = P 2 V 2

BOYLES LAW A 2. 00 Liter balloon is filled with helium gas at a pressure of 760 torr. What will be the volume of the balloon if the pressure is lowered to 608 torr? 1 2

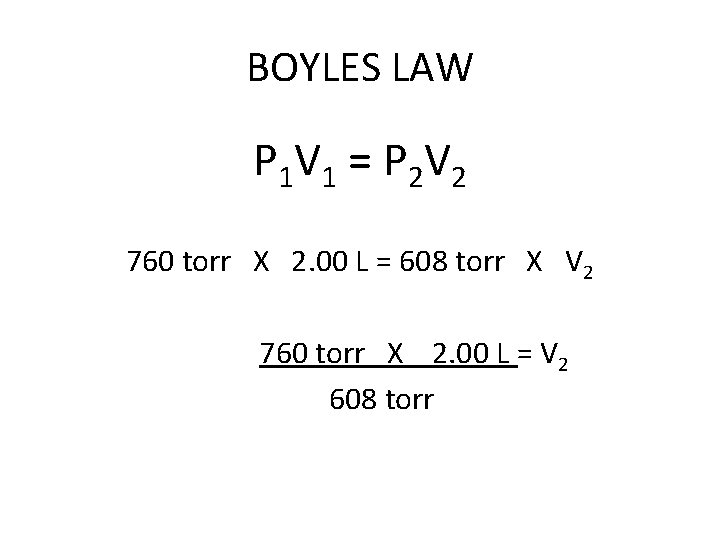
BOYLES LAW P 1 V 1 = P 2 V 2 760 torr X 2. 00 L = 608 torr X V 2

BOYLES LAW P 1 V 1 = P 2 V 2 760 torr X 2. 00 L = 608 torr X V 2 760 torr X 2. 00 L = V 2 608 torr

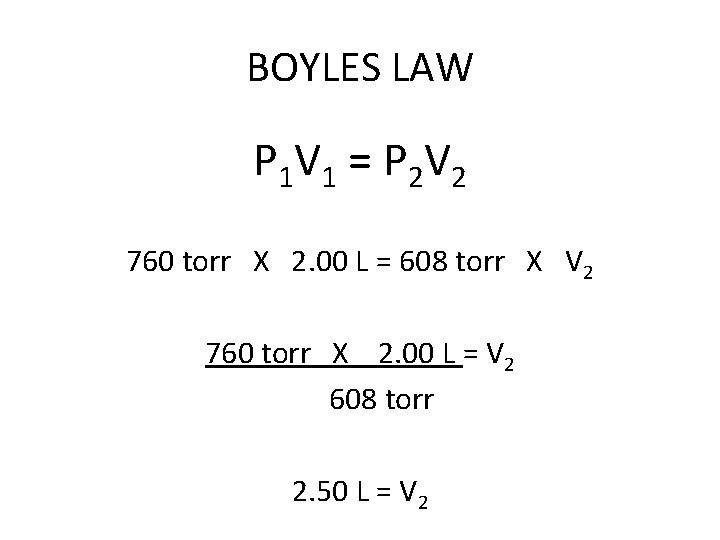
BOYLES LAW P 1 V 1 = P 2 V 2 760 torr X 2. 00 L = 608 torr X V 2 760 torr X 2. 00 L = V 2 608 torr 2. 50 L = V 2

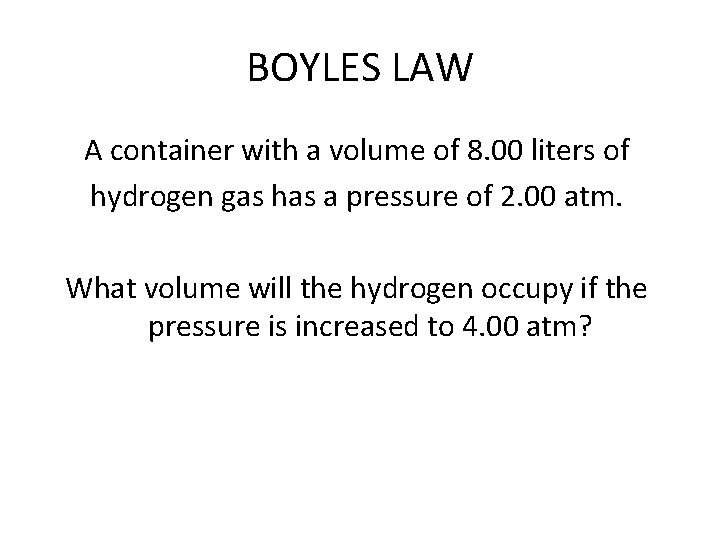
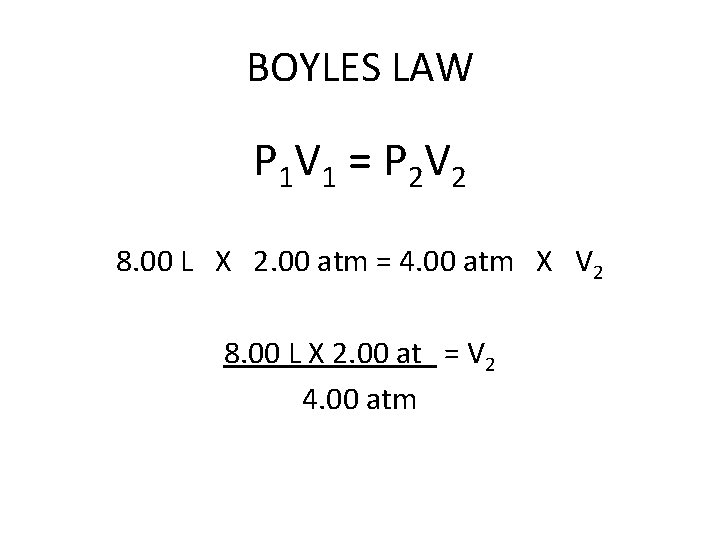
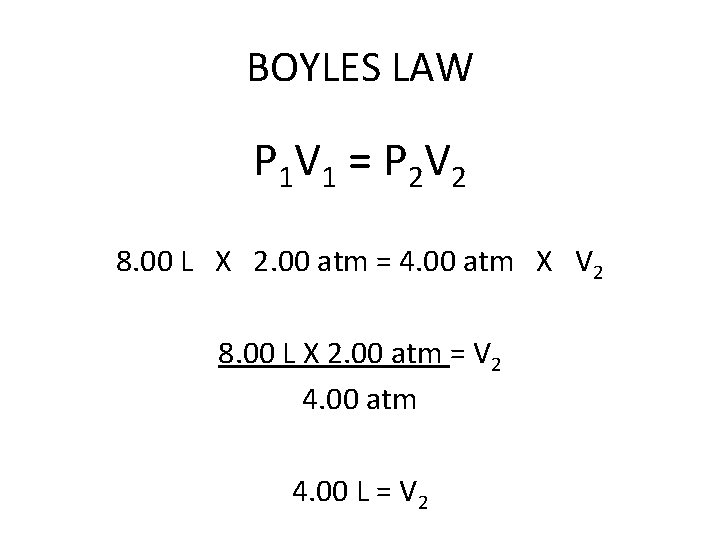
BOYLES LAW A container with a volume of 8. 00 liters of hydrogen gas has a pressure of 2. 00 atm. What volume will the hydrogen occupy if the pressure is increased to 4. 00 atm?

BOYLES LAW P 1 V 1 = P 2 V 2 8. 00 L X 2. 00 atm = 4. 00 atm X V 2

BOYLES LAW P 1 V 1 = P 2 V 2 8. 00 L X 2. 00 atm = 4. 00 atm X V 2 8. 00 L X 2. 00 at = V 2 4. 00 atm

BOYLES LAW P 1 V 1 = P 2 V 2 8. 00 L X 2. 00 atm = 4. 00 atm X V 2 8. 00 L X 2. 00 atm = V 2 4. 00 atm 4. 00 L = V 2

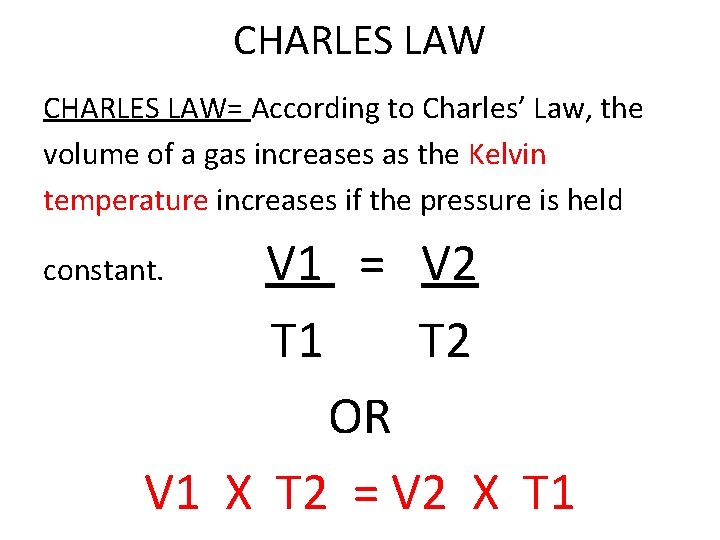
CHARLES LAW= According to Charles’ Law, the volume of a gas increases as the Kelvin temperature increases if the pressure is held V 1 = V 2 T 1 T 2 OR V 1 X T 2 = V 2 X T 1 constant.

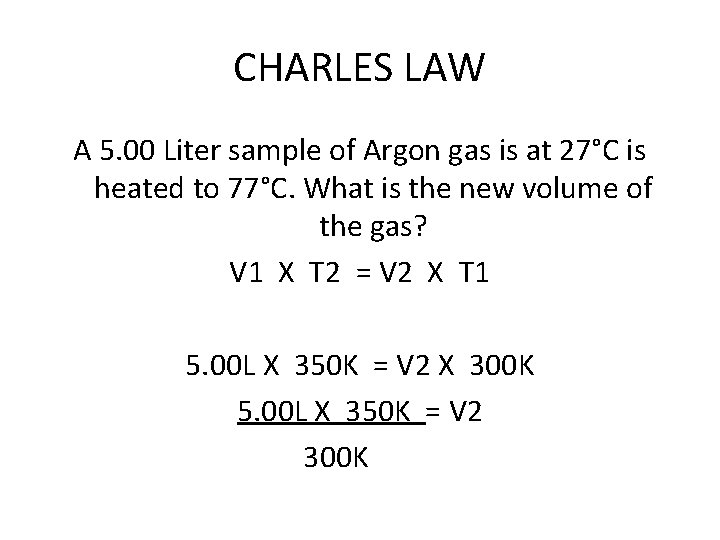
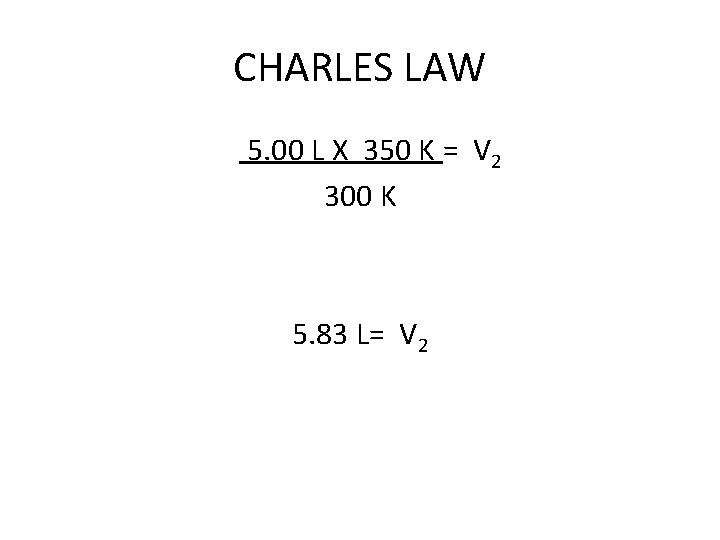
CHARLES LAW A 5. 00 Liter sample of Argon gas is at 27°C is heated to 77°C. What is the new volume of the gas? V 1 X T 2 = V 2 X T 1 5. 00 L X 350 K = V 2 X 300 K 5. 00 L X 350 K = V 2 300 K

CHARLES LAW 5. 00 L X 350 K = V 2 300 K 5. 83 L= V 2

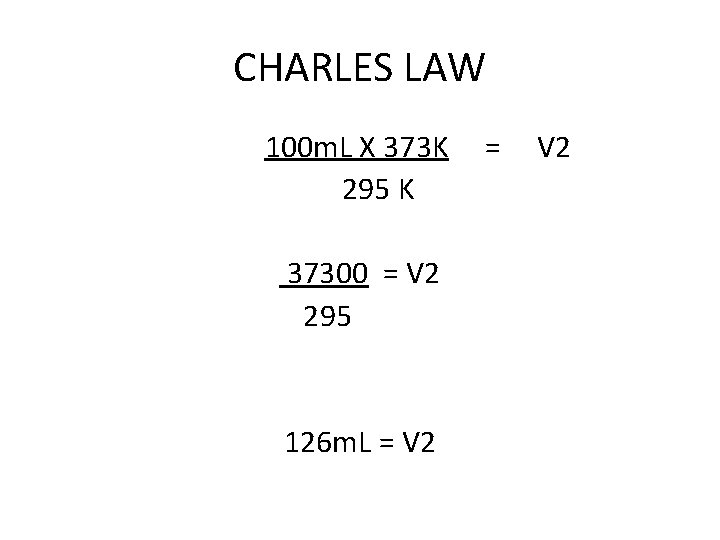
CHARLES LAW 100 m. L of a gas is heated from 22°C to 100°C. what is the new volume of the gas? V 1 X T 2 = V 2 X T 1 100 m. L x 373 K = V 2 X 295 K

CHARLES LAW 100 m. L X 373 K 295 K 37300 = V 2 295 126 m. L = V 2

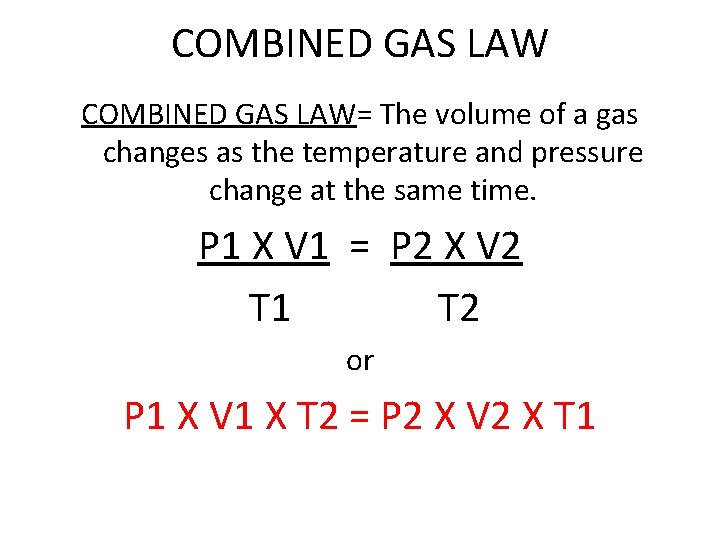
COMBINED GAS LAW= The volume of a gas changes as the temperature and pressure change at the same time. P 1 X V 1 = P 2 X V 2 T 1 T 2 or P 1 X V 1 X T 2 = P 2 X V 2 X T 1

COMBINED GAS LAW A 10. 0 liter weather balloon is filled with helium at a temperature of 20° C and a pressure of 740 torr. The balloon rises in the atmosphere to a point where the temperature is -30° C and the pressure is 350 torr. What is the new volume of the balloon? P 1 X V 1 X T 2 = P 2 X V 2 X T 1

COMBINED GAS LAW P 1 X V 1 X T 2 = P 2 X V 2 X T 1 740 t x 10 L x 243 K = 350 t x V 2 x 293 K 1798200 = 102550 x V 2 1798200 = V 2 102550 17. 5 L = V 2

COMBINED GAS LAW A 25. 0 m. L sample of carbon dioxide is at 25°C and 800 torr of pressure. To what Kelvin temperature must the gas be heated to increase its volume to 30. 0 m. L at a pressure of 900 torr? P 1 X V 1 X T 2 = P 2 X V 2 X T 1

COMBINED GAS LAW 800 t x 25 m. L x T 2 = 900 t X 30. 0 m. L x 298 K 20000 x T 2 = 8046000 20000 T 2 = 402 K or 129 C

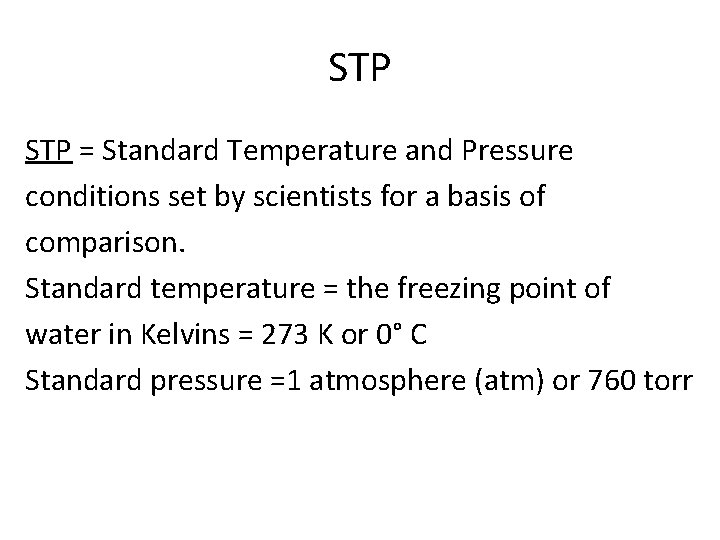
STP = Standard Temperature and Pressure conditions set by scientists for a basis of comparison. Standard temperature = the freezing point of water in Kelvins = 273 K or 0° C Standard pressure =1 atmosphere (atm) or 760 torr

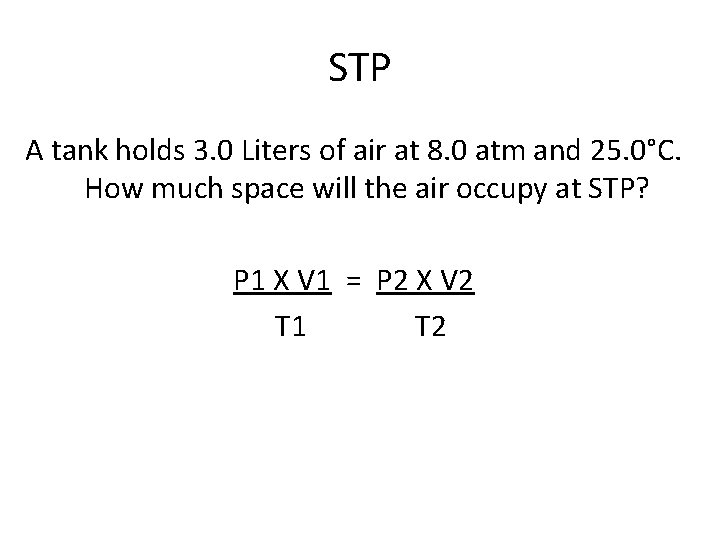
STP A tank holds 3. 0 Liters of air at 8. 0 atm and 25. 0°C. How much space will the air occupy at STP? P 1 X V 1 = P 2 X V 2 T 1 T 2

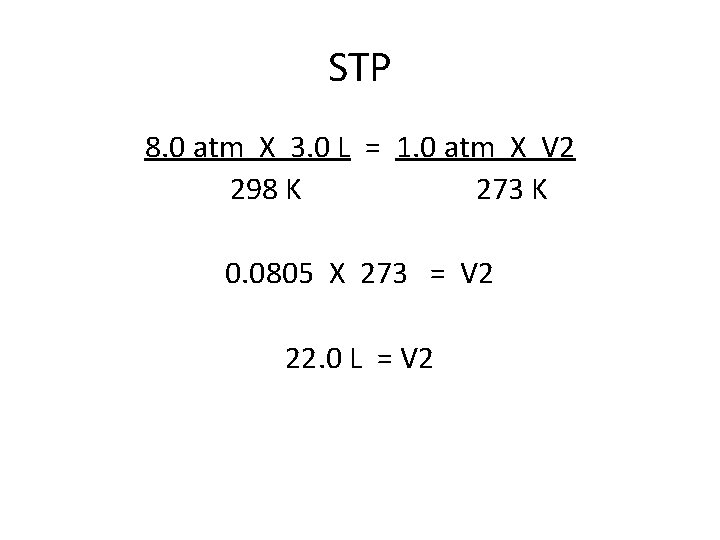
STP 8. 0 atm X 3. 0 L = 1. 0 atm X V 2 298 K 273 K 0. 0805 X 273 = V 2 22. 0 L = V 2

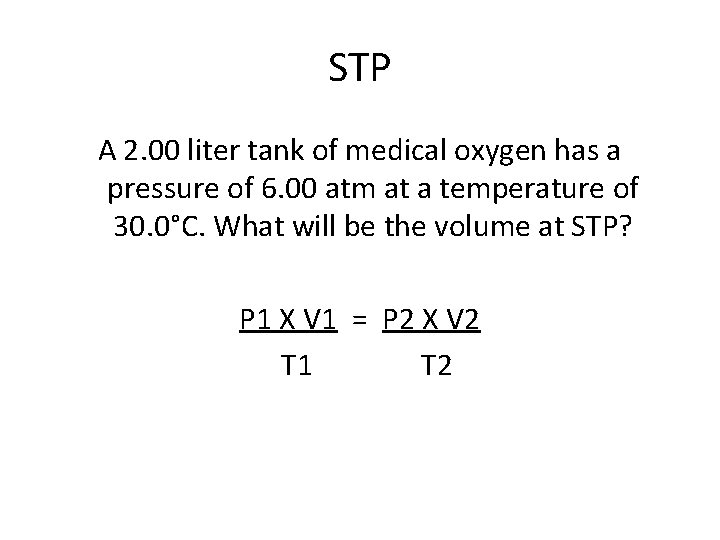
STP A 2. 00 liter tank of medical oxygen has a pressure of 6. 00 atm at a temperature of 30. 0°C. What will be the volume at STP? P 1 X V 1 = P 2 X V 2 T 1 T 2

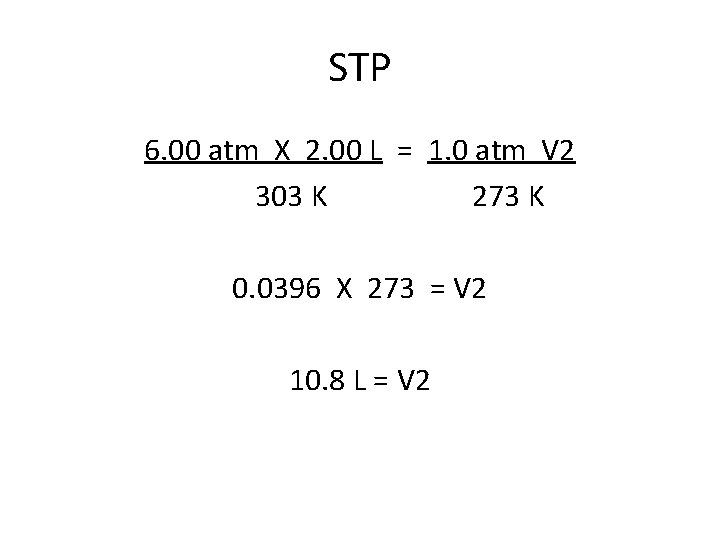
STP 6. 00 atm X 2. 00 L = 1. 0 atm V 2 303 K 273 K 0. 0396 X 273 = V 2 10. 8 L = V 2

IDEAL GAS LAW P X V =n X R X T P = PRESSURE = measured in atmospheres (atm) V = VOLUME = measured in Liters n = number of moles R = gas constant 0. 082 T = TEMPERATURE = measured in Kelvins

IDEAL GAS LAW Calculate the volume 3. 00 moles of a gas will occupy at 24. 0 °C and 1. 00 atm. P X V =n X R X T

IDEAL GAS LAW 1. 00 atm X V = 3. 00 moles X 0. 082 X 297 1. 00 atm V = 73. 1 L

IDEAL GAS LAW How many moles of gas would be present in a gas trapped within a 100. 0 m. L vessel at 25. 0 °C at a pressure of 2. 50 atmospheres? P X V =n X R X T

IDEAL GAS LAW 2. 50 atm X 0. 1 L = n X 0. 082 X 298 K 0. 25 = n X 24. 4 0. 25 = n 24. 4 0. 0102 = n
 Mikael ferm
Mikael ferm Nature and nature's laws lay hid in night meaning
Nature and nature's laws lay hid in night meaning Facts about montesquieu
Facts about montesquieu State boyle’s law.
State boyle’s law. Module 3 topic 1 laws of nature
Module 3 topic 1 laws of nature Present simple general truths examples
Present simple general truths examples Module 3 topic 1 laws of nature
Module 3 topic 1 laws of nature Gas laws crash course
Gas laws crash course Direct vs indirect relationship chemistry
Direct vs indirect relationship chemistry What are the empirical gas laws
What are the empirical gas laws Gas laws calculations
Gas laws calculations Bourdon gauge gas law
Bourdon gauge gas law Different gas laws
Different gas laws How hot air balloons operate gas laws
How hot air balloons operate gas laws Conceptual gas law questions
Conceptual gas law questions Mathematical formula for charles law
Mathematical formula for charles law Chapter 14 the behavior of gases worksheet answer key
Chapter 14 the behavior of gases worksheet answer key Boyle's gas law formula
Boyle's gas law formula Different gas laws
Different gas laws Combined gas law worksheet
Combined gas law worksheet Section 13.2 the combined gas law and avogadro's principle
Section 13.2 the combined gas law and avogadro's principle Boyle's law states that
Boyle's law states that Empirical gas laws
Empirical gas laws Ap chemistry gas laws
Ap chemistry gas laws Gas laws graphic organizer
Gas laws graphic organizer Gas laws hot air balloon
Gas laws hot air balloon Combined gas law example in real life
Combined gas law example in real life Kmt gas laws
Kmt gas laws Gas law formula
Gas law formula Empirical gas laws
Empirical gas laws Avogadro's law relationship
Avogadro's law relationship The gas laws
The gas laws Solid volume definite or indefinite
Solid volume definite or indefinite Nature nature controversy
Nature nature controversy Ideal gas vs perfect gas
Ideal gas vs perfect gas Imaginary gas
Imaginary gas Differences between ideal gas and real gas
Differences between ideal gas and real gas Ideal gas vs perfect gas
Ideal gas vs perfect gas Bhopal gas tragedy reason
Bhopal gas tragedy reason Gas leaked in bhopal gas tragedy
Gas leaked in bhopal gas tragedy