Gases Properties of Gases Gas Laws pressure volume



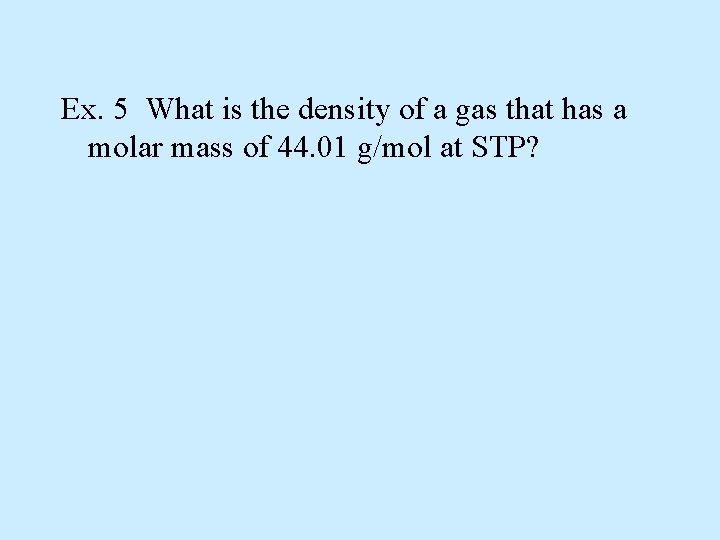











- Slides: 68

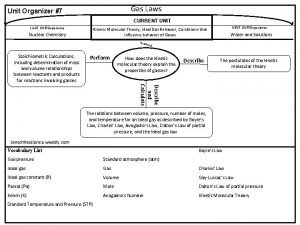
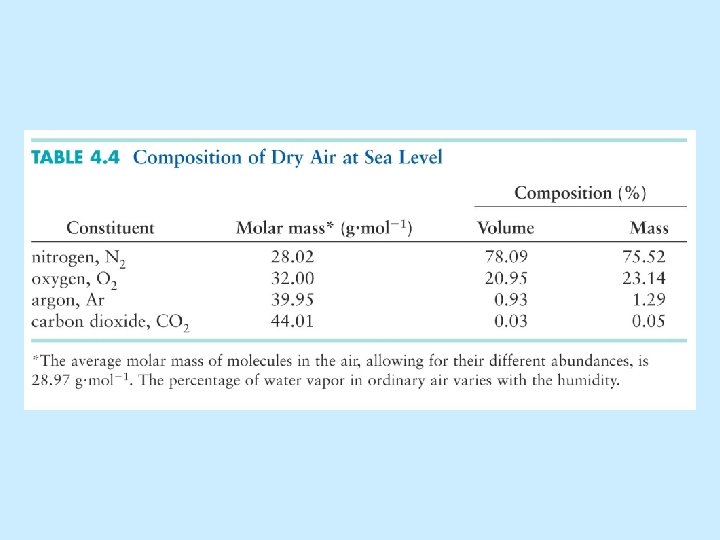
Gases • Properties of Gases • Gas Laws (pressure, volume, temperature, moles) • Gases in Chemical Reactions • The Kinetic Model of Gases


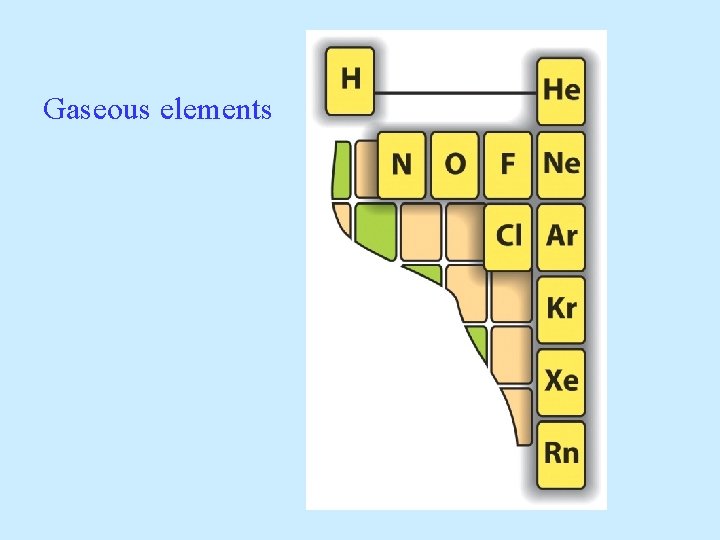
Gaseous elements

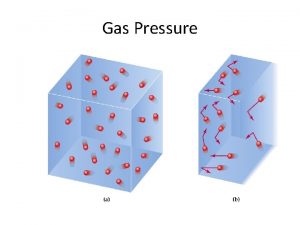
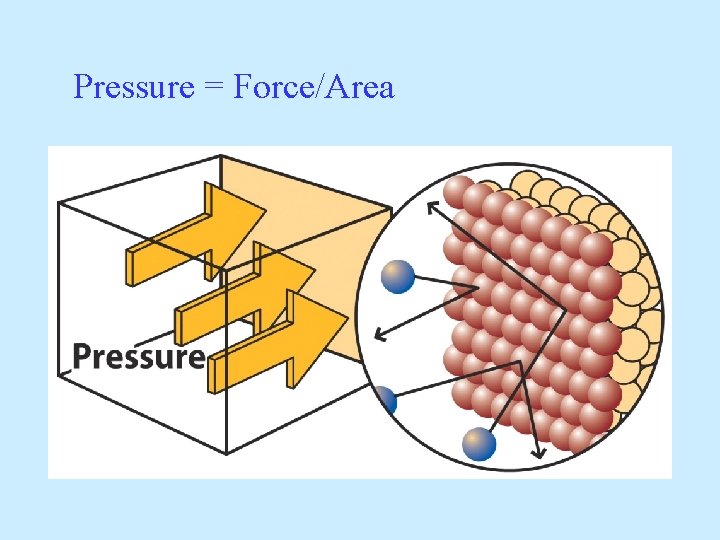
Pressure = Force/Area


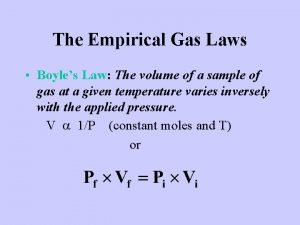
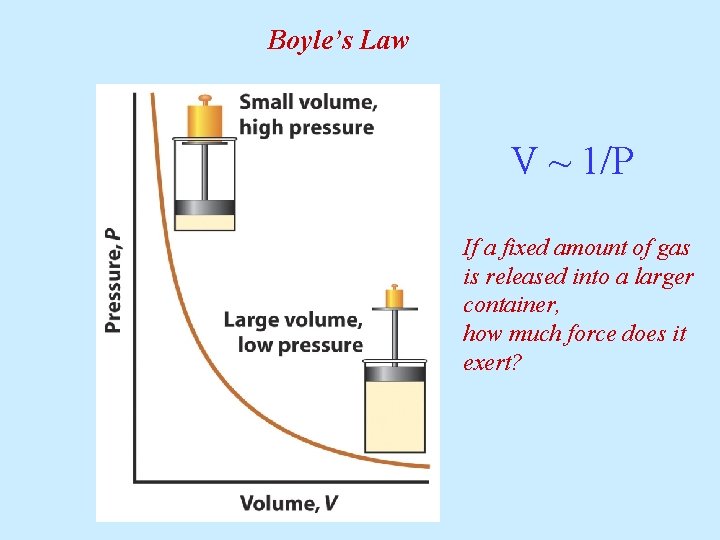
Boyle’s Law V ~ 1/P If a fixed amount of gas is released into a larger container, how much force does it exert?

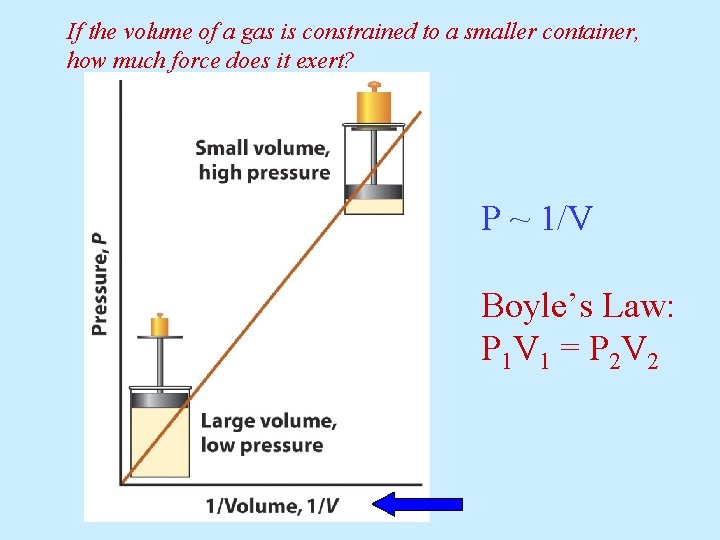
If the volume of a gas is constrained to a smaller container, how much force does it exert? P ~ 1/V Boyle’s Law: P 1 V 1 = P 2 V 2

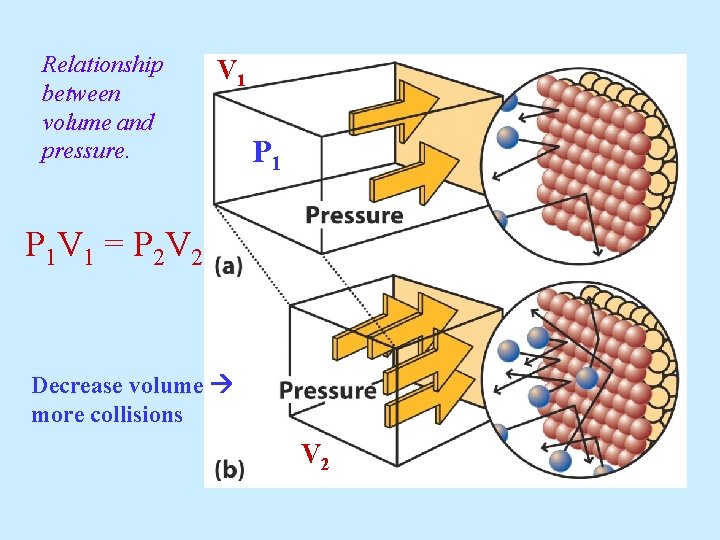
Relationship between volume and pressure. V 1 P 1 V 1 = P 2 V 2 Decrease volume more collisions V 2

• Ex. 1 A sample of gas occupies 21 liters at a pressure of 2. 2 atm. What would be the volume if the pressure was increased to 6. 2 atm? • Ex. 2 A sample of O 2 occupies 10. 0 L at 785 torr. At what pressure would it occupy 14. 5 L?

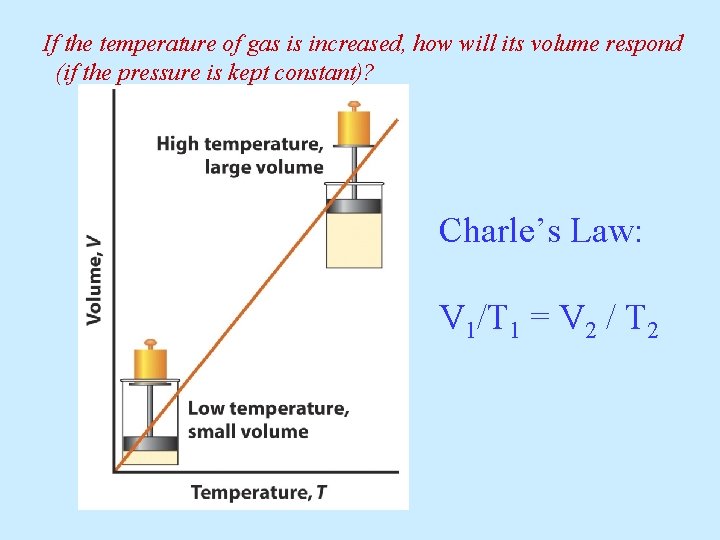
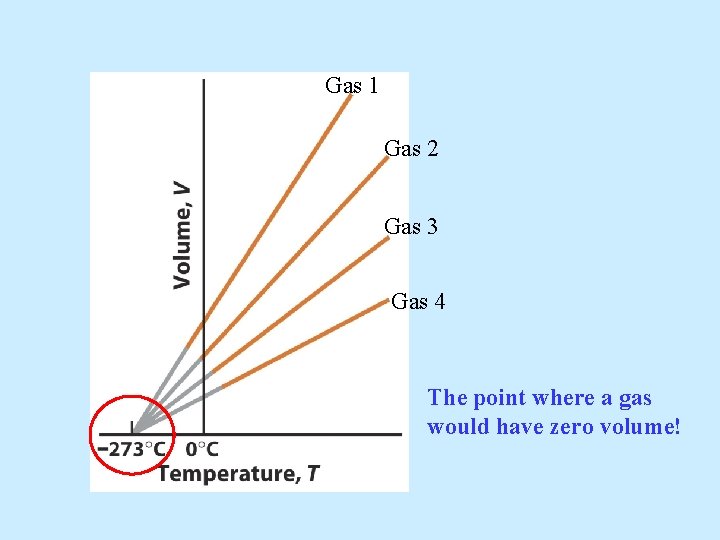
If the temperature of gas is increased, how will its volume respond (if the pressure is kept constant)? Charle’s Law: V 1/T 1 = V 2 / T 2

Gas 1 Gas 2 Gas 3 Gas 4 The point where a gas would have zero volume!

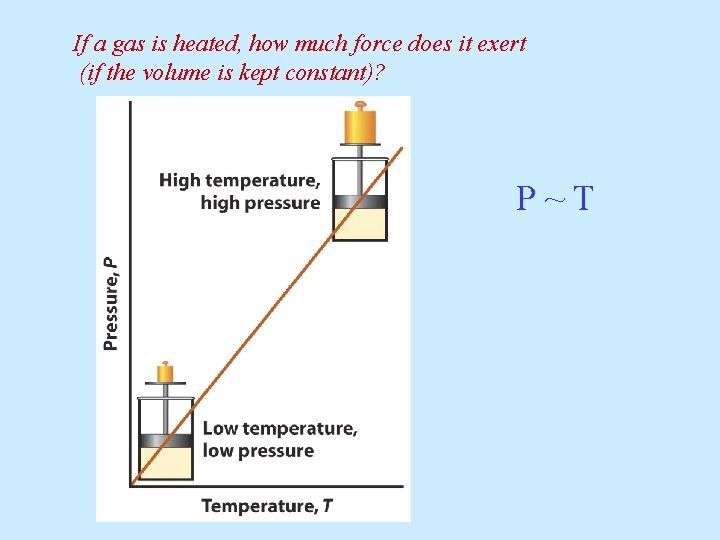
If a gas is heated, how much force does it exert (if the volume is kept constant)? P~T

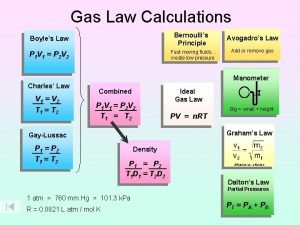
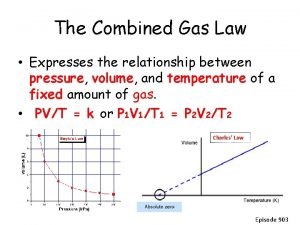
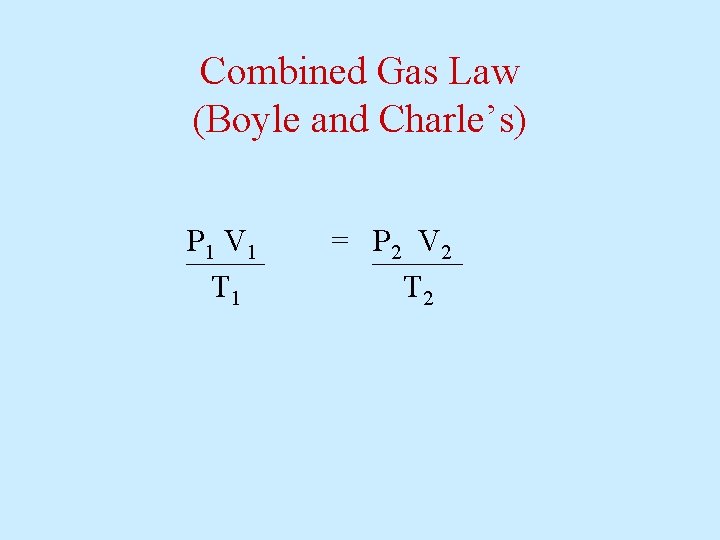
Combined Gas Law (Boyle and Charle’s) P 1 V 1 T 1 = P 2 V 2 T 2

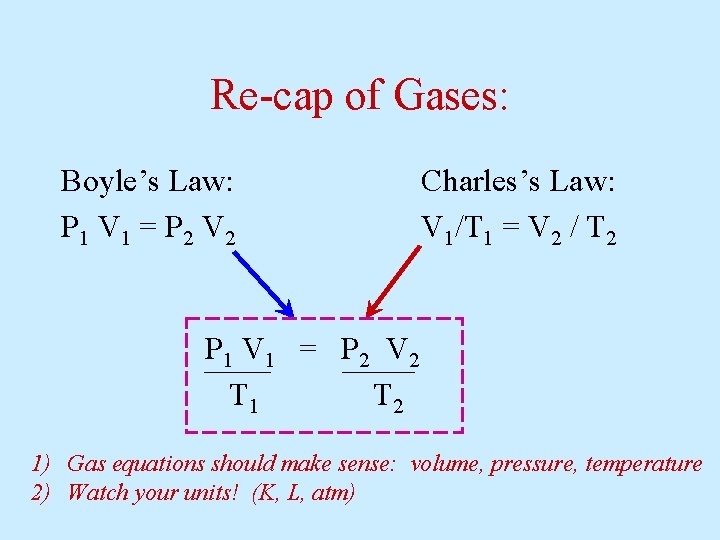
Re-cap of Gases: Boyle’s Law: P 1 V 1 = P 2 V 2 Charles’s Law: V 1/T 1 = V 2 / T 2 P 1 V 1 = P 2 V 2 T 1 T 2 1) Gas equations should make sense: volume, pressure, temperature 2) Watch your units! (K, L, atm)

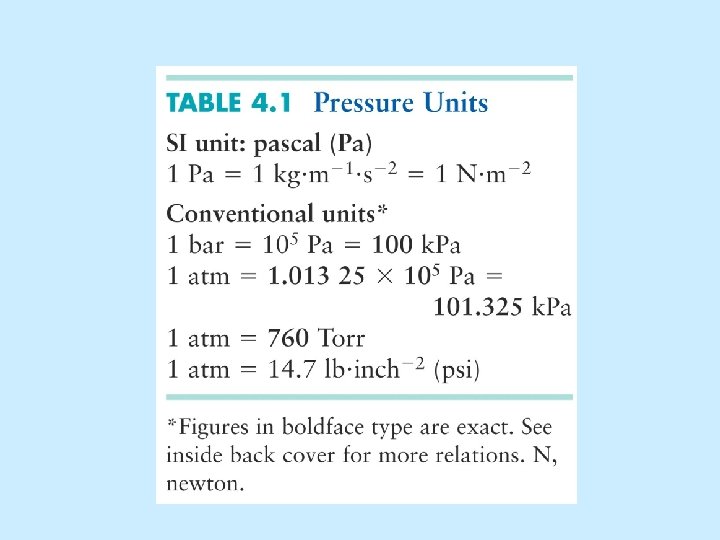
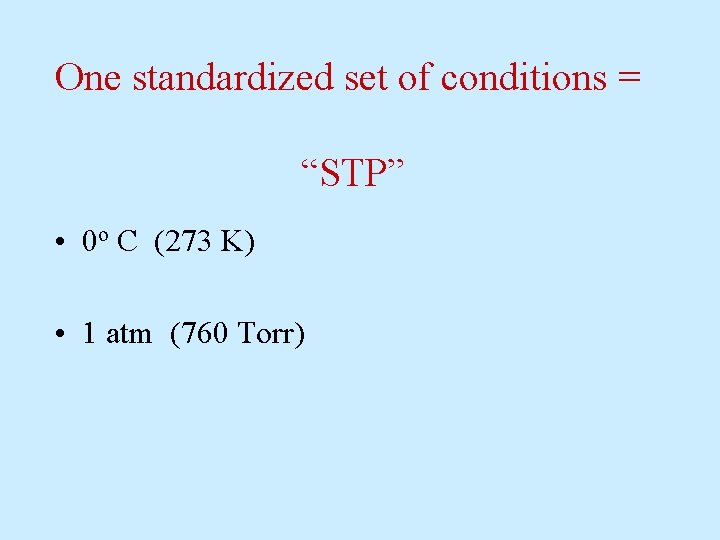
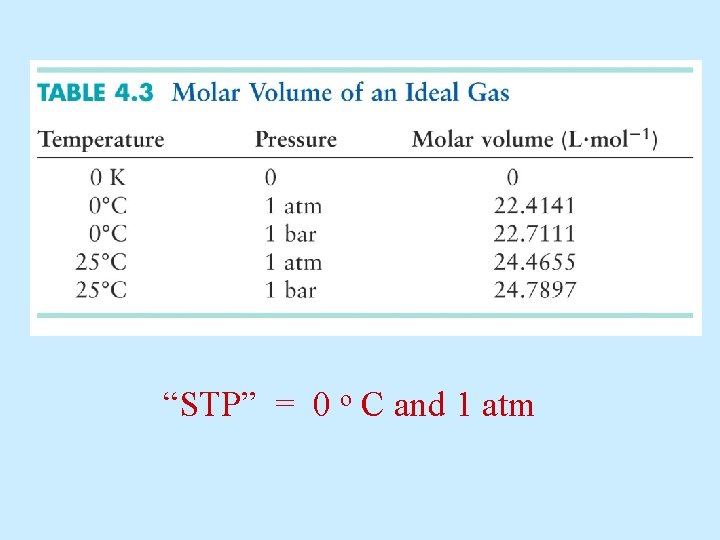
One standardized set of conditions = “STP” • 0 o C (273 K) • 1 atm (760 Torr)

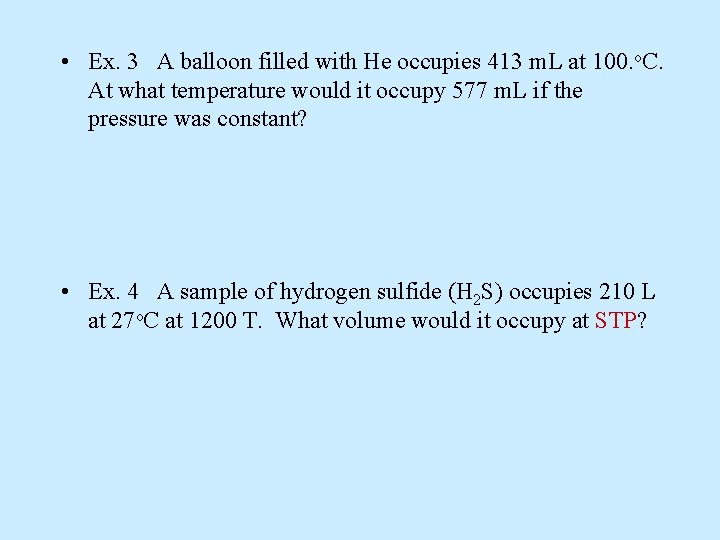
• Ex. 3 A balloon filled with He occupies 413 m. L at 100. o. C. At what temperature would it occupy 577 m. L if the pressure was constant? • Ex. 4 A sample of hydrogen sulfide (H 2 S) occupies 210 L at 27 o. C at 1200 T. What volume would it occupy at STP?

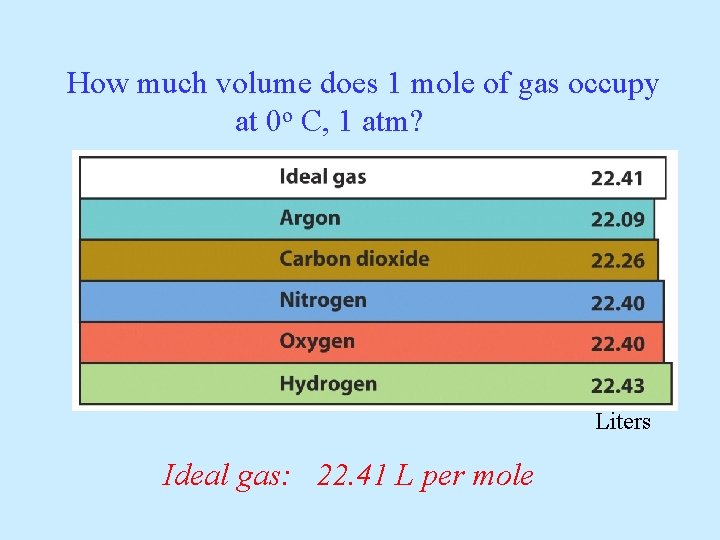
How much volume does 1 mole of gas occupy at 0 o C, 1 atm? If one mole of Argon occupies 22. 1 L, how much volume will be occupied by: one mole CO 2 one mole N 2 one mole O 2 one mole H 2

How much volume does 1 mole of gas occupy at 0 o C, 1 atm? Liters Ideal gas: 22. 41 L per mole
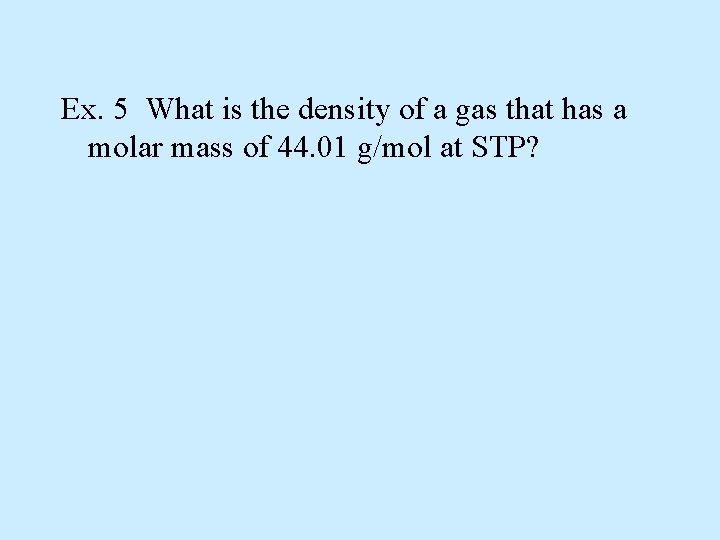
Ex. 5 What is the density of a gas that has a molar mass of 44. 01 g/mol at STP?

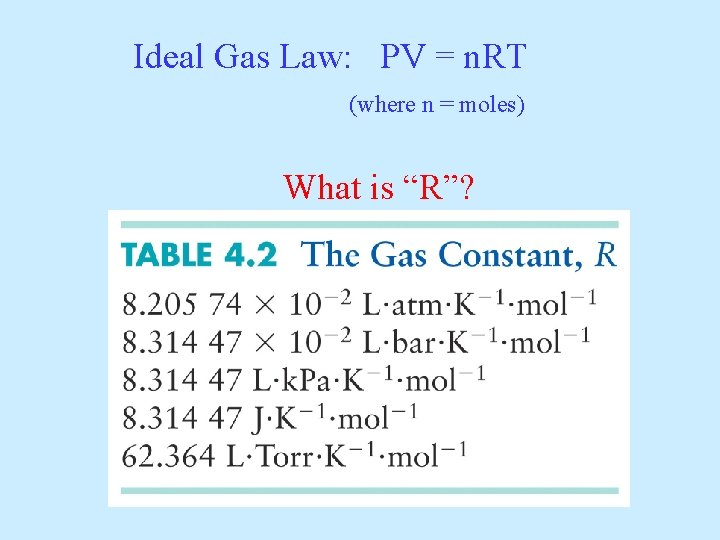
Ideal Gas Law: PV = n. RT (where n = moles) What is “R”?

“STP” = 0 o C and 1 atm

• Ex. 6 What is the volume of a balloon filled with 32. 02 grams of Helium when the atmospheric pressure is 722 torr and the temperature is 40 o C? • Ex. 7 The Goodyear blimp must be inflated with Helium prior to a football game. Its volume is 7601 ft 3. How many grams of He are needed for a pressure of 740 torr at 22 o C? (1 ft 3 = 28. 3 L)

• Ex. 9 A 0. 723 g sample of a gas occupies 176 m. L at 100. o C and 750. torr. What is its molar mass?

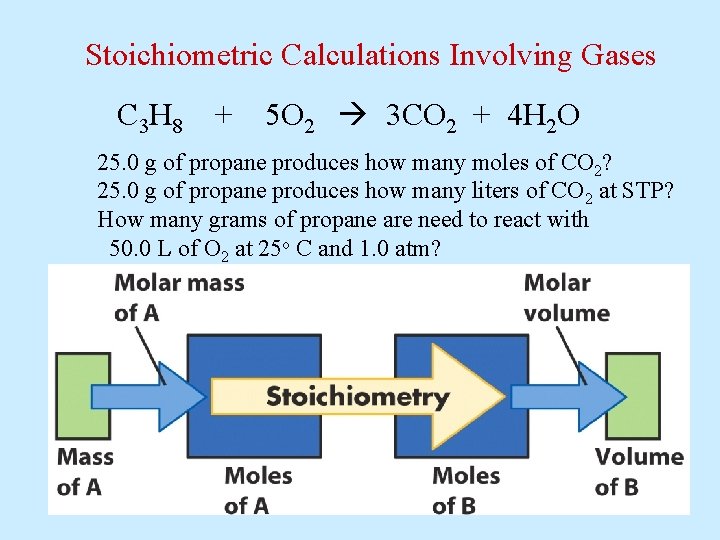
Stoichiometric Calculations Involving Gases C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O 25. 0 g of propane produces how many moles of CO 2? 25. 0 g of propane produces how many liters of CO 2 at STP? How many grams of propane are need to react with 50. 0 L of O 2 at 25 o C and 1. 0 atm?

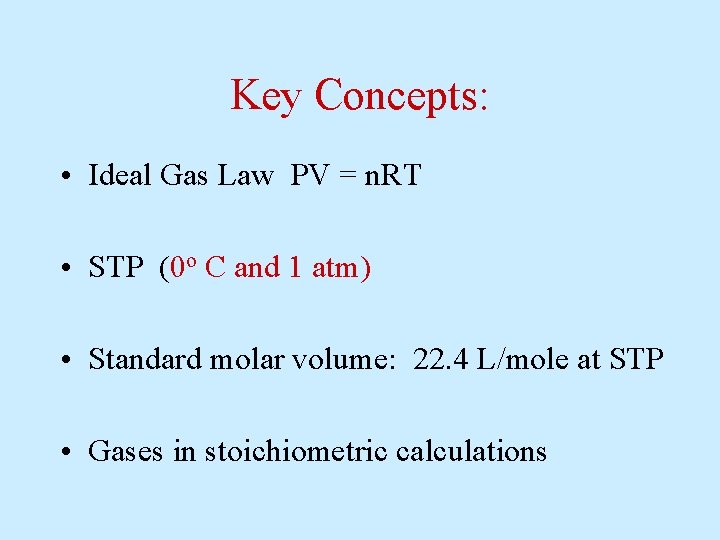
Key Concepts: • Ideal Gas Law PV = n. RT • STP (0 o C and 1 atm) • Standard molar volume: 22. 4 L/mole at STP • Gases in stoichiometric calculations

An airbag inflates in less than 50 msec by the reaction of Na. N 3 to produce Na and nitrogen gas: Na. N 3 Na + N 2 The volume of the airbag is about 30 L when inflated, and it is filled to a pressure of 1. 4 atm. How many grams of Na. N 3 must be used for each air bag? The molar mass of Na. N 3 is 65. 1 g/mole. Assume the process occurs at room temperature.

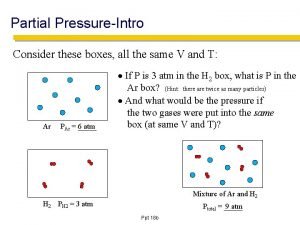
Gas mixtures • Dalton’s Law of partial pressures The total pressure of a mixture of gases equals the sum of the pressures that each would exert if it were present alone PT=P 1+P 2+P 3+…. Pn Exercise: A gaseous mixture is made from 6. 00 g oxygen and 9. 00 g methane placed in a 15 L vessel at 0 o. C. What is the partial pressure of each gas and the total pressure in the vessel?

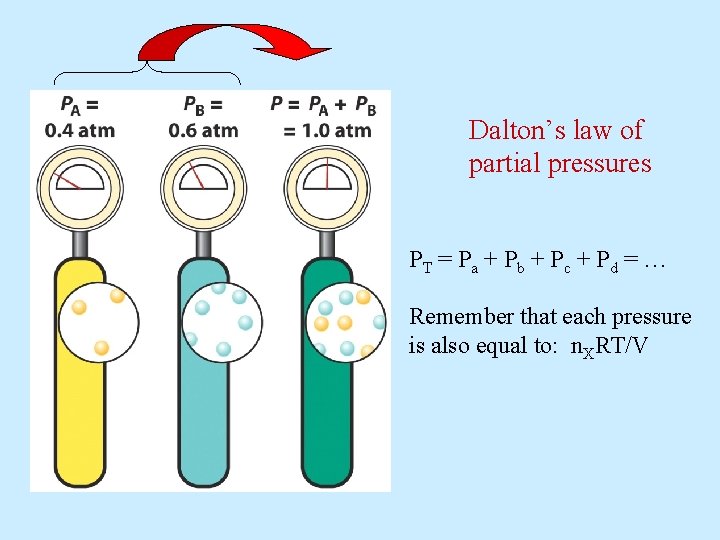
Dalton’s law of partial pressures PT = P a + P b + P c + P d = … Remember that each pressure is also equal to: n. XRT/V

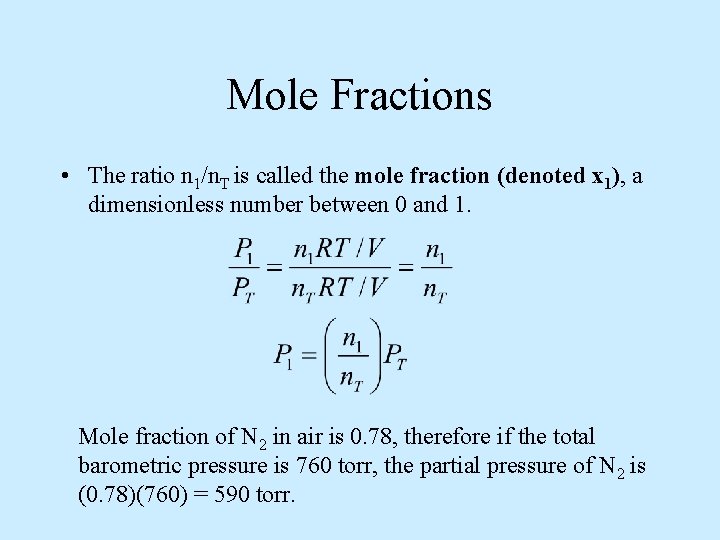
Mole Fractions • The ratio n 1/n. T is called the mole fraction (denoted x 1), a dimensionless number between 0 and 1. Mole fraction of N 2 in air is 0. 78, therefore if the total barometric pressure is 760 torr, the partial pressure of N 2 is (0. 78)(760) = 590 torr.

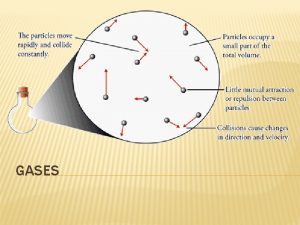
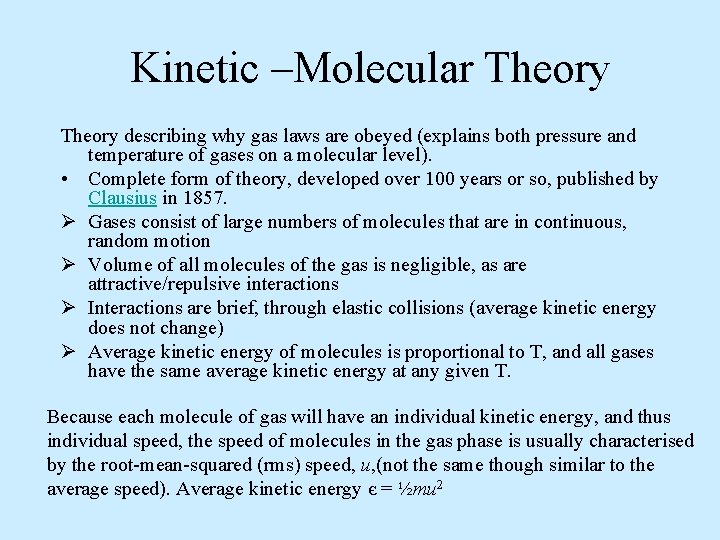
Kinetic –Molecular Theory describing why gas laws are obeyed (explains both pressure and temperature of gases on a molecular level). • Complete form of theory, developed over 100 years or so, published by Clausius in 1857. Ø Gases consist of large numbers of molecules that are in continuous, random motion Ø Volume of all molecules of the gas is negligible, as are attractive/repulsive interactions Ø Interactions are brief, through elastic collisions (average kinetic energy does not change) Ø Average kinetic energy of molecules is proportional to T, and all gases have the same average kinetic energy at any given T. Because each molecule of gas will have an individual kinetic energy, and thus individual speed, the speed of molecules in the gas phase is usually characterised by the root-mean-squared (rms) speed, u, (not the same though similar to the average speed). Average kinetic energy є = ½mu 2

Application to Gas Laws • Increasing V at constant T: Constant T means that u is unchanged. But if V is increased the likelihood of collision with the walls decreases, thus the pressure decreases (Boyle’s Law) • Increasing T at constant V: Increasing T increases u, increasing collisional frequency with the walls, thus the pressure increases (Ideal Gas Equation).

• Ex. 10 A 20. 5 L bulb contains 0. 200 moles of methane, 0. 300 moles of hydrogen, and 0. 400 moles of nitrogen at 20. 0 o C. It is stinky and explosive. What is the pressure inside the bulb? How much pressure is contributed by each of the three gases? • Ex. 12 One tank of gas contains 5. 00 L of N 2 at 32. 0 atm. A second tank contains 3. 00 L of O 2 at 24. 0 atm. What pressure is attained when the valve between the tanks is opened?

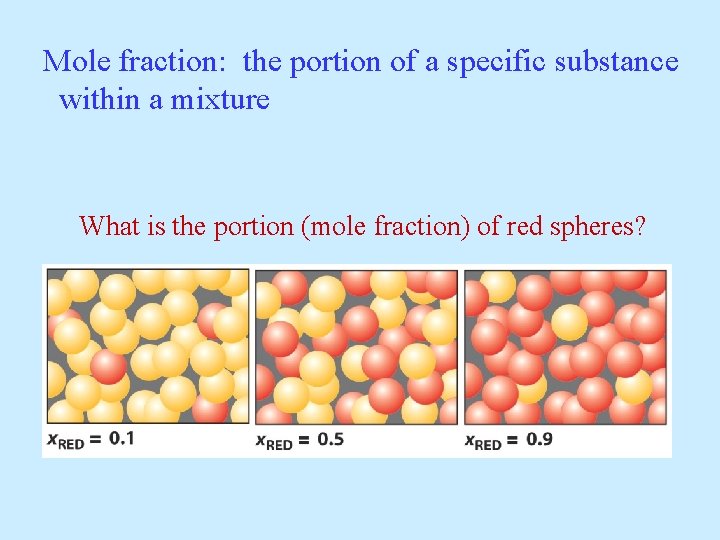
Mole fraction: the portion of a specific substance within a mixture What is the portion (mole fraction) of red spheres?

Kinetic model of gases each dot is one gas molecule

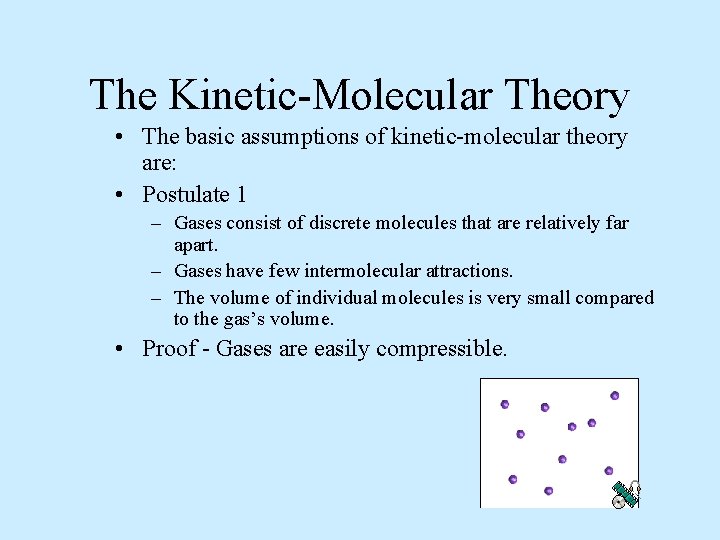
The Kinetic-Molecular Theory • The basic assumptions of kinetic-molecular theory are: • Postulate 1 – Gases consist of discrete molecules that are relatively far apart. – Gases have few intermolecular attractions. – The volume of individual molecules is very small compared to the gas’s volume. • Proof - Gases are easily compressible.

The Kinetic-Molecular Theory • Postulate 2 – Gas molecules are in constant, random, straight line motion with varying velocities. • Proof - Brownian motion displays molecular motion.

The Kinetic-Molecular Theory • Postulate 3 – Gas molecules have elastic collisions with themselves and the container. – Total energy is conserved during a collision. • Proof - A sealed, confined gas exhibits no pressure drop over time.

The Kinetic-Molecular Theory • Postulate 4 – The kinetic energy of the molecules is proportional to the absolute temperature. – The average kinetic energies of molecules of different gases are equal at a given temperature. • Proof - Brownian motion increases as temperature increases.

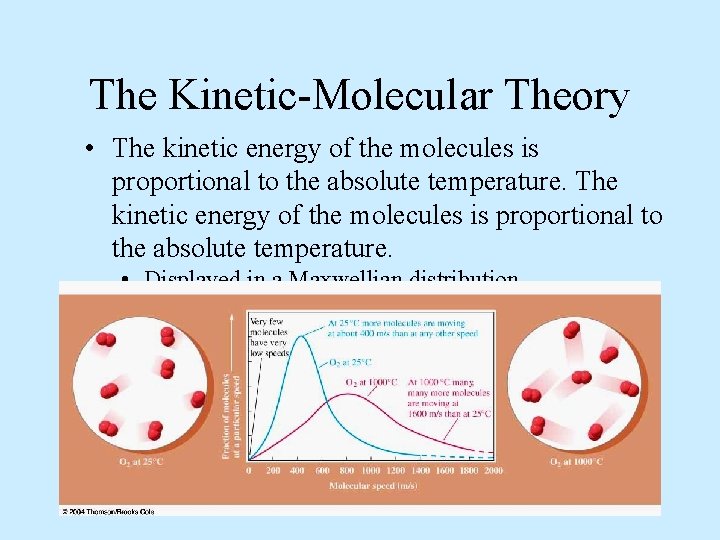
The Kinetic-Molecular Theory • The kinetic energy of the molecules is proportional to the absolute temperature. • Displayed in a Maxwellian distribution.

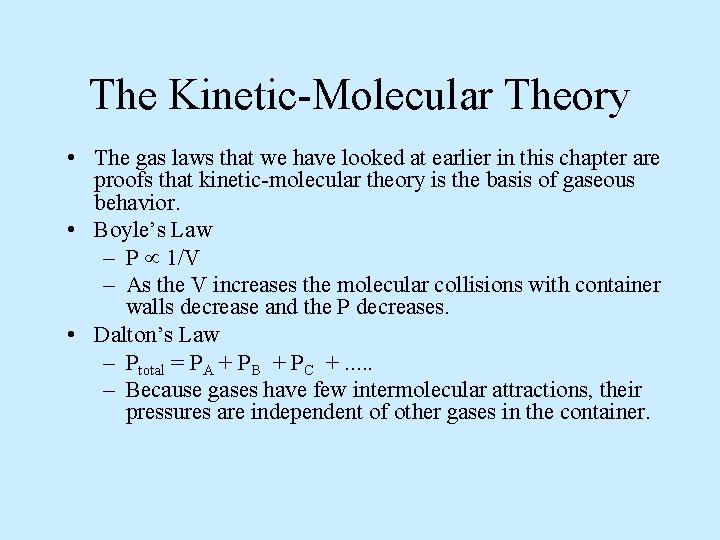
The Kinetic-Molecular Theory • The gas laws that we have looked at earlier in this chapter are proofs that kinetic-molecular theory is the basis of gaseous behavior. • Boyle’s Law – P 1/V – As the V increases the molecular collisions with container walls decrease and the P decreases. • Dalton’s Law – Ptotal = PA + PB + PC +. . . – Because gases have few intermolecular attractions, their pressures are independent of other gases in the container.

• Charles’ Law – V T – An increase in temperature raises the molecular velocities, thus the V increases to keep the P constant.

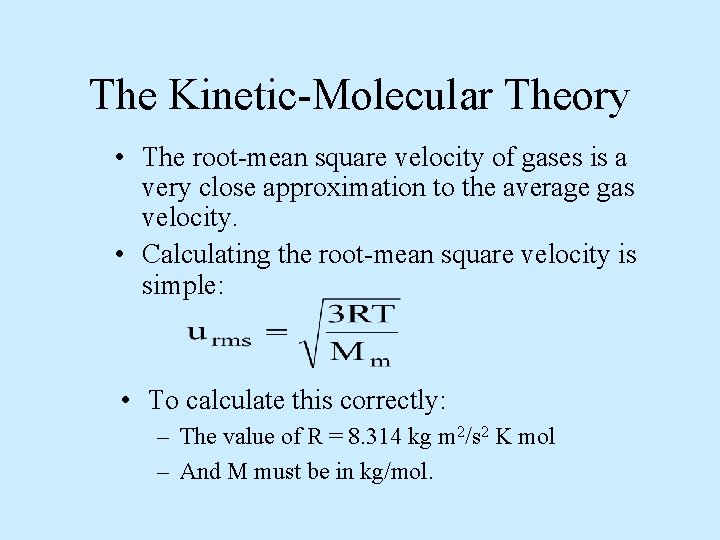
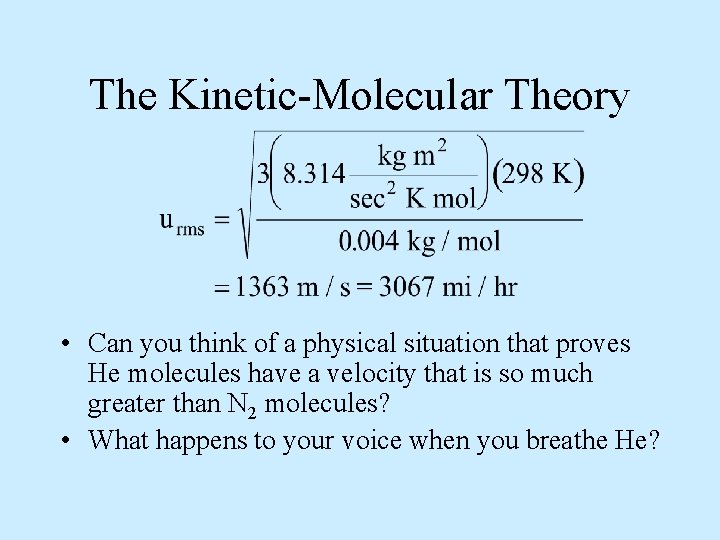
The Kinetic-Molecular Theory • The root-mean square velocity of gases is a very close approximation to the average gas velocity. • Calculating the root-mean square velocity is simple: • To calculate this correctly: – The value of R = 8. 314 kg m 2/s 2 K mol – And M must be in kg/mol.

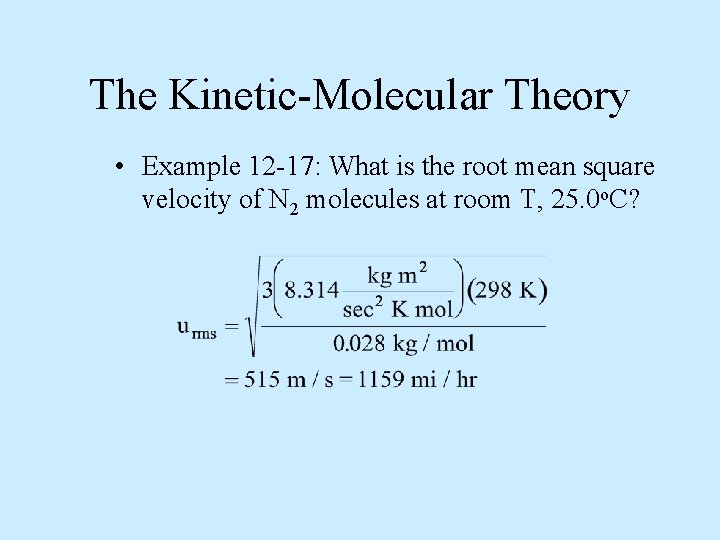
The Kinetic-Molecular Theory • Example 12 -17: What is the root mean square velocity of N 2 molecules at room T, 25. 0 o. C?

The Kinetic-Molecular Theory • What is the root mean square velocity of He atoms at room T, 25. 0 o. C?

The Kinetic-Molecular Theory • Can you think of a physical situation that proves He molecules have a velocity that is so much greater than N 2 molecules? • What happens to your voice when you breathe He?

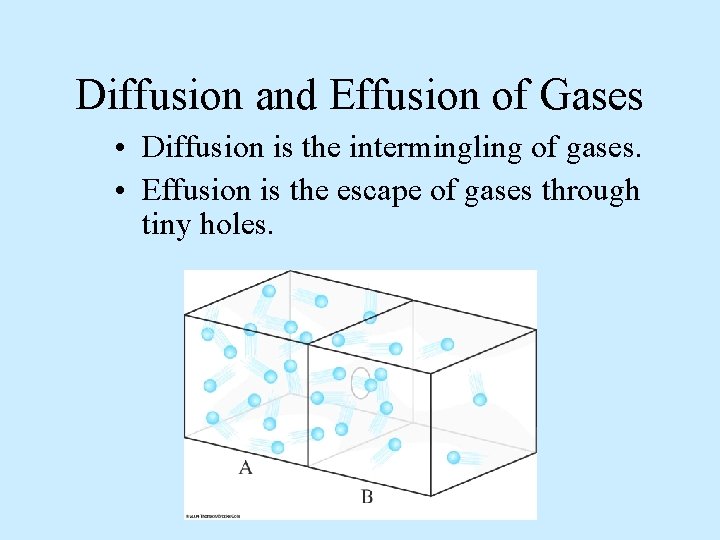
Diffusion and Effusion of Gases • Diffusion is the intermingling of gases. • Effusion is the escape of gases through tiny holes.

Diffusion and Effusion of Gases • This is a demonstration of diffusion.

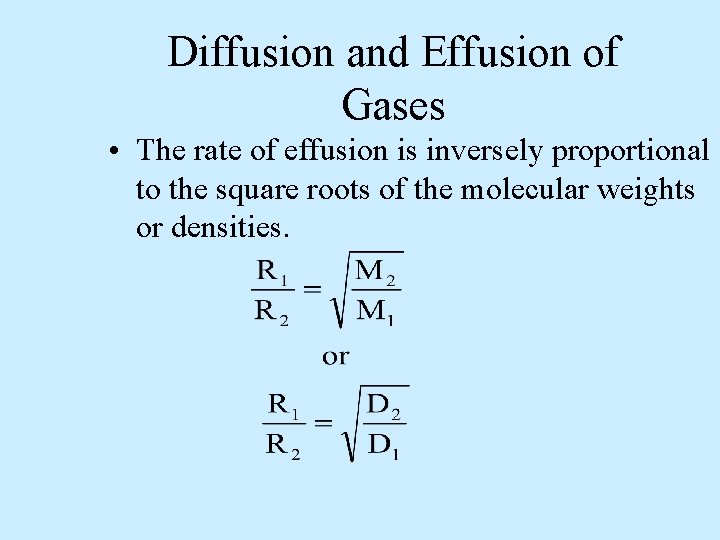
Diffusion and Effusion of Gases • The rate of effusion is inversely proportional to the square roots of the molecular weights or densities.

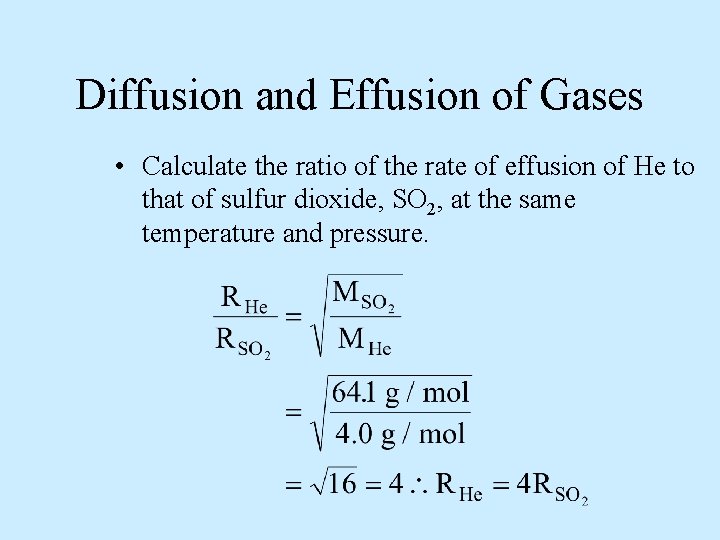
Diffusion and Effusion of Gases • Calculate the ratio of the rate of effusion of He to that of sulfur dioxide, SO 2, at the same temperature and pressure.

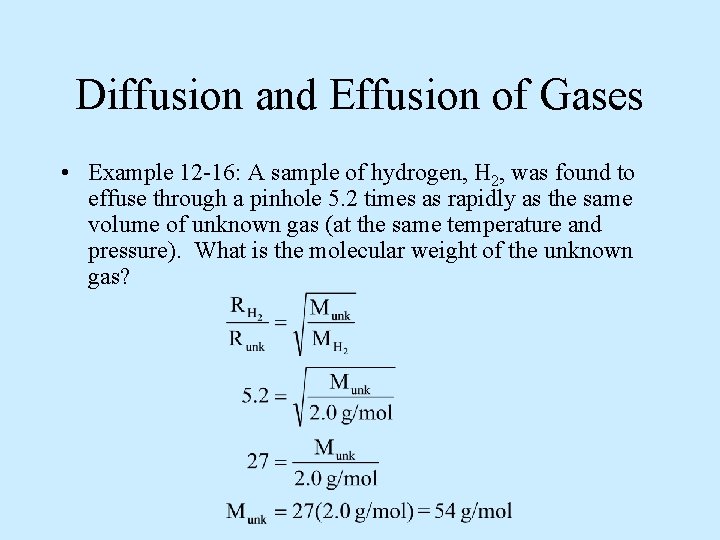
Diffusion and Effusion of Gases • Example 12 -16: A sample of hydrogen, H 2, was found to effuse through a pinhole 5. 2 times as rapidly as the same volume of unknown gas (at the same temperature and pressure). What is the molecular weight of the unknown gas?

Real Gases: Deviations from Ideality • • • Real gases behave ideally at ordinary temperatures and pressures. At low temperatures and high pressures real gases do not behave ideally. The reasons for the deviations from ideality are: 1. The molecules are very close to one another, thus their volume is important. 2. The molecular interactions also become important.

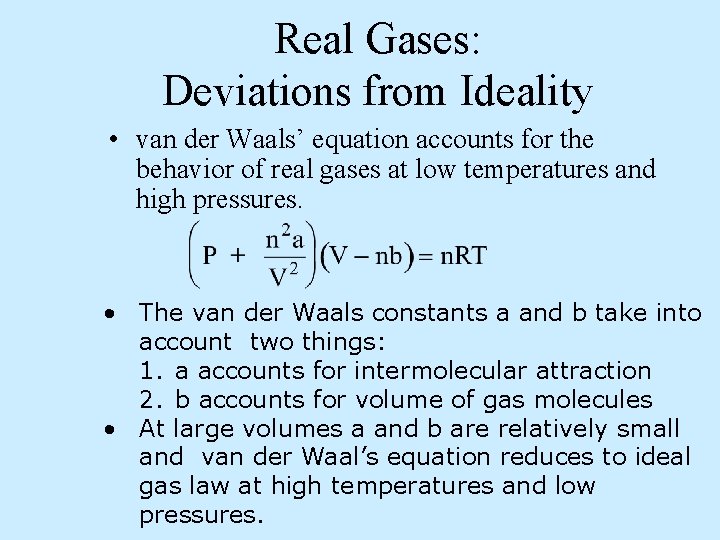
Real Gases: Deviations from Ideality • van der Waals’ equation accounts for the behavior of real gases at low temperatures and high pressures. • The van der Waals constants a and b take into account two things: 1. a accounts for intermolecular attraction 2. b accounts for volume of gas molecules • At large volumes a and b are relatively small and van der Waal’s equation reduces to ideal gas law at high temperatures and low pressures.

Real Gases: Deviations from Ideality • What are the intermolecular forces in gases that cause them to deviate from ideality? 1. For nonpolar gases the attractive forces are London Forces 2. For polar gases the attractive forces are dipole-dipole attractions or hydrogen bonds.

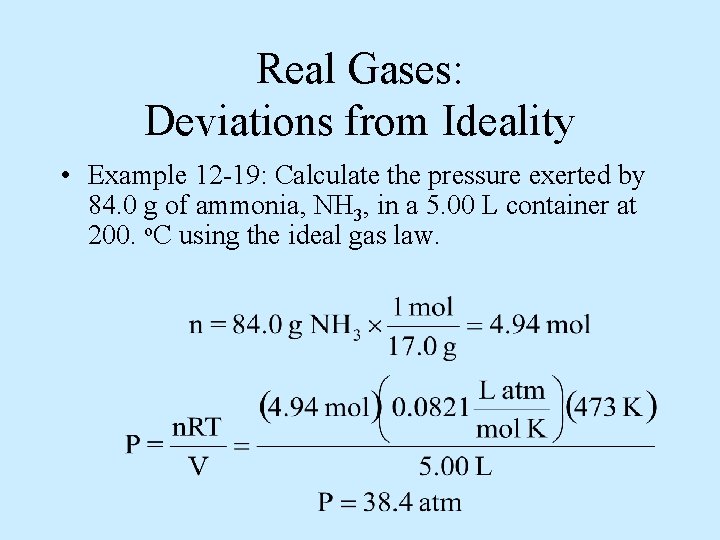
Real Gases: Deviations from Ideality • Example 12 -19: Calculate the pressure exerted by 84. 0 g of ammonia, NH 3, in a 5. 00 L container at 200. o. C using the ideal gas law.

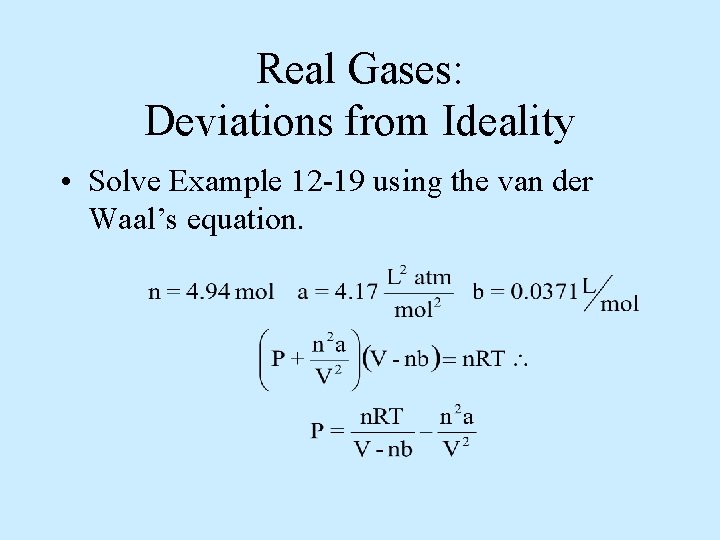
Real Gases: Deviations from Ideality • Solve Example 12 -19 using the van der Waal’s equation.

Real Gases: Deviations from Ideality

“Real” gases • Deviations from ideal behavior as gases are exposed to: – high pressures – low temperatures (What happens at high pressures and low temperatures? ? ? )

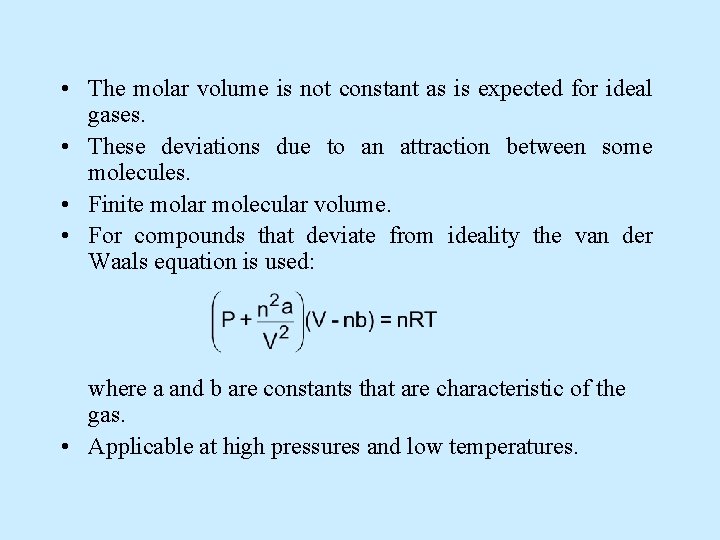
• The molar volume is not constant as is expected for ideal gases. • These deviations due to an attraction between some molecules. • Finite molar molecular volume. • For compounds that deviate from ideality the van der Waals equation is used: where a and b are constants that are characteristic of the gas. • Applicable at high pressures and low temperatures.

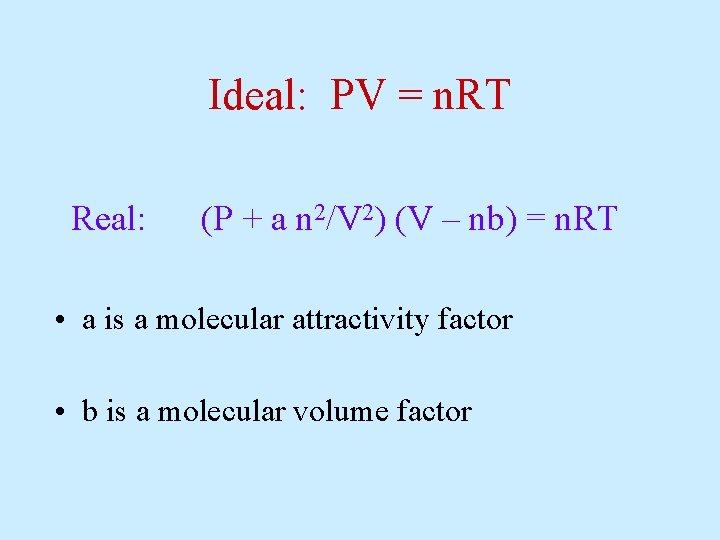
Ideal: PV = n. RT Real: (P + a n 2/V 2) (V – nb) = n. RT • a is a molecular attractivity factor • b is a molecular volume factor

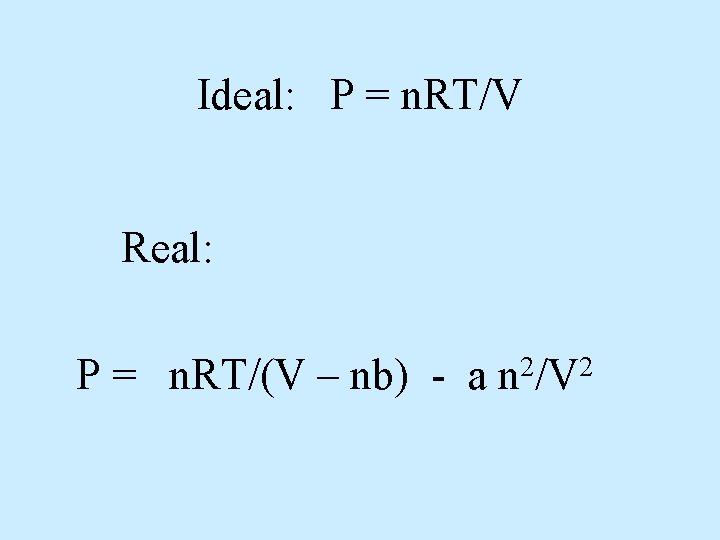
Ideal: P = n. RT/V Real: P = n. RT/(V – nb) - a 2 2 n /V

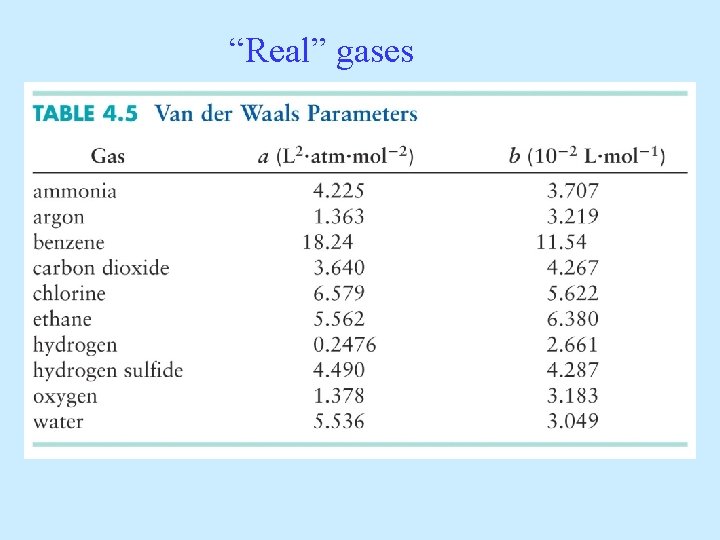
“Real” gases

Ex. 13 Calculate the pressure exerted by 5. 00 moles of NH 3 in a 1. 00 L vessel at 25. 0 o. C assuming ideal and non-ideal behavior.

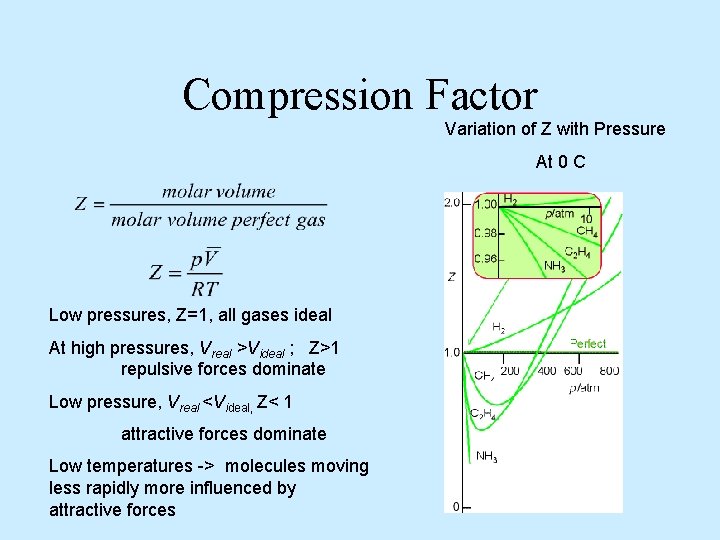
Compression Factor Variation of Z with Pressure At 0 C Low pressures, Z=1, all gases ideal At high pressures, Vreal >Videal ; Z>1 repulsive forces dominate Low pressure, Vreal <Videal, Z< 1 attractive forces dominate Low temperatures -> molecules moving less rapidly more influenced by attractive forces

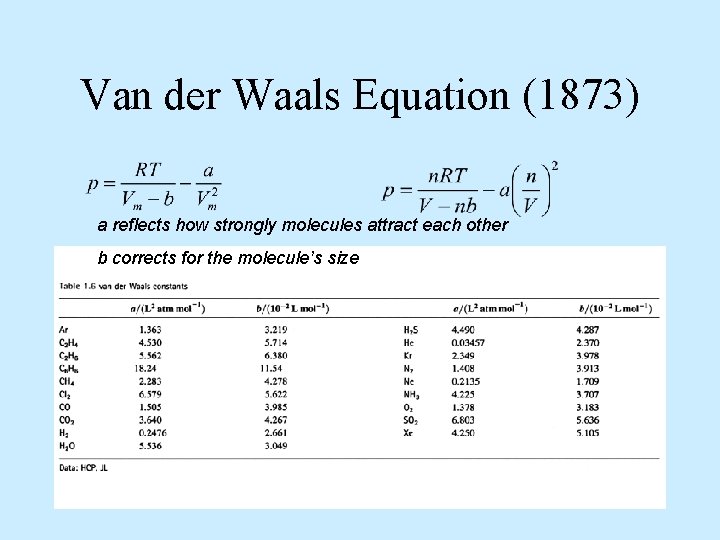
Van der Waals Equation (1873) a reflects how strongly molecules attract each other b corrects for the molecule’s size

“Derivation” of vdw Eq. State Repulsive interactions cause molecules to behave as impenetrable spheres Molecules restricted to smaller volume V-nb, where nb is volume molecules take up Pressure depends on frequency of collisions with walls and force of each collision – both reduced by attractive forces proportional to molar concentration (n/V) Pressure is then reduced according to a(n/V)2

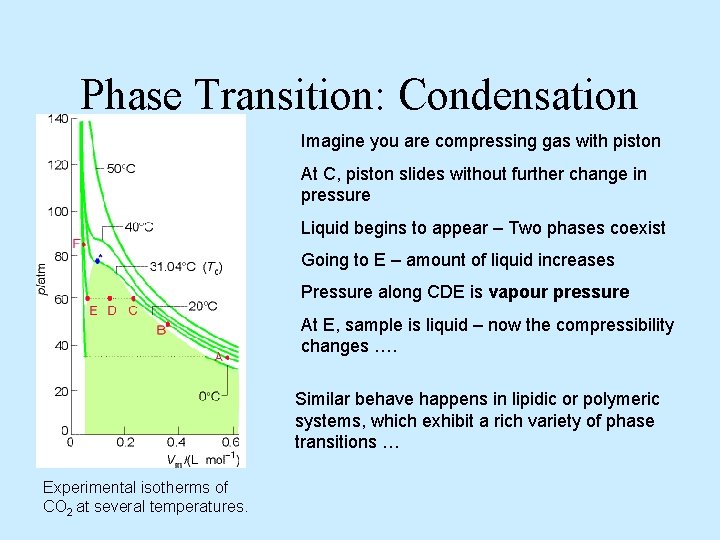
Phase Transition: Condensation Imagine you are compressing gas with piston At C, piston slides without further change in pressure Liquid begins to appear – Two phases coexist Going to E – amount of liquid increases Pressure along CDE is vapour pressure At E, sample is liquid – now the compressibility changes …. Similar behave happens in lipidic or polymeric systems, which exhibit a rich variety of phase transitions … Experimental isotherms of CO 2 at several temperatures.

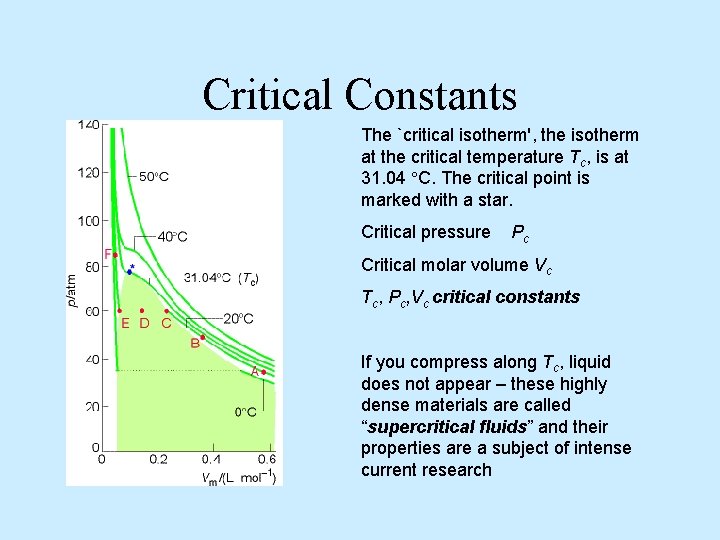
Critical Constants The `critical isotherm', the isotherm at the critical temperature Tc, is at 31. 04 C. The critical point is marked with a star. Critical pressure Pc Critical molar volume Vc Tc, Pc, Vc critical constants If you compress along Tc, liquid does not appear – these highly dense materials are called “supercritical fluids” and their properties are a subject of intense current research

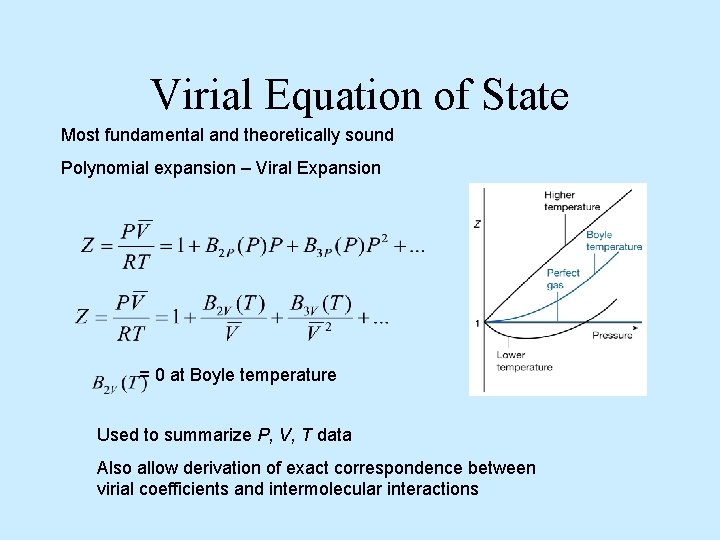
Virial Equation of State Most fundamental and theoretically sound Polynomial expansion – Viral Expansion = 0 at Boyle temperature Used to summarize P, V, T data Also allow derivation of exact correspondence between virial coefficients and intermolecular interactions
 Facts about montesquieu
Facts about montesquieu Gas pressure
Gas pressure Do gases exert pressure on whatever surrounds them
Do gases exert pressure on whatever surrounds them Gas laws crash course
Gas laws crash course Volume and pressure direct or indirect
Volume and pressure direct or indirect Empirical gas laws
Empirical gas laws Combined gas law
Combined gas law Bourdon gauge gas law
Bourdon gauge gas law Different gas laws
Different gas laws Charles law
Charles law Conceptual gas law questions
Conceptual gas law questions Which gas laws are inversely proportional
Which gas laws are inversely proportional Chapter 13 gases answer key
Chapter 13 gases answer key A gas occupies 473 cm3 at 36°c. find its volume at 94°c
A gas occupies 473 cm3 at 36°c. find its volume at 94°c Different gas laws
Different gas laws Combined gas laws
Combined gas laws Chapter 13 gases
Chapter 13 gases Boyle's law graph
Boyle's law graph Empirical gas laws
Empirical gas laws Ap chemistry gas laws
Ap chemistry gas laws Gas law graphic organizer
Gas law graphic organizer Gas laws hot air balloon
Gas laws hot air balloon Formula for boyle's law
Formula for boyle's law Kmt gas laws
Kmt gas laws Gas laws formula
Gas laws formula Empirical gas law
Empirical gas law Combined gas law
Combined gas law Gas law
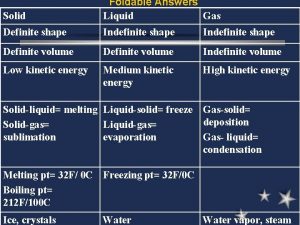
Gas law Is gas definite or indefinite
Is gas definite or indefinite Solids liquids and gases section 2 properties of fluids
Solids liquids and gases section 2 properties of fluids Properties of solids and liquids
Properties of solids and liquids Properties of gas
Properties of gas Four properties of gas
Four properties of gas 5 properties of gases
5 properties of gases Properties of gases
Properties of gases Noble gases characteristics
Noble gases characteristics Physical properties of gases
Physical properties of gases Why do gases have low densities
Why do gases have low densities Chemical properties of noble gases
Chemical properties of noble gases Properties of solids liquids and gases
Properties of solids liquids and gases Properties of a gas
Properties of a gas Properties of gas
Properties of gas List 2 of the important properties of gases
List 2 of the important properties of gases Insall salvati ratio
Insall salvati ratio Pressure support vs pressure control
Pressure support vs pressure control Continuous bedside pressure mapping
Continuous bedside pressure mapping Intrapulmonary pressure vs intrapleural pressure
Intrapulmonary pressure vs intrapleural pressure Starling's equation
Starling's equation Partial pressure
Partial pressure Intrapleural pressure
Intrapleural pressure Regional metamorphism
Regional metamorphism ütube
ütube Stream line equation
Stream line equation Oncotic vs hydrostatic pressure
Oncotic vs hydrostatic pressure Interstitial fluid hydrostatic pressure
Interstitial fluid hydrostatic pressure Low oncotic pressure
Low oncotic pressure Hydrostatic oncotic pressure
Hydrostatic oncotic pressure Metamorphism
Metamorphism How is blood pressure regulated
How is blood pressure regulated How to find partial pressure from total pressure
How to find partial pressure from total pressure Vc+ vs prvc
Vc+ vs prvc High pressure and low pressure
High pressure and low pressure Tiefdruckgebiet
Tiefdruckgebiet Relation between pressure and kinetic energy of gas
Relation between pressure and kinetic energy of gas Gaya yang besarnya 50 dyne menimbulkan tekanan
Gaya yang besarnya 50 dyne menimbulkan tekanan What gas law relates pressure and temperature
What gas law relates pressure and temperature Partial pressure gas law
Partial pressure gas law A gas has a pressure of 699 mmhg at 40
A gas has a pressure of 699 mmhg at 40 Gas pressure units
Gas pressure units