The Gas Laws Direct vs Indirect Relationships Directly

- Slides: 12

The Gas Laws

Direct vs Indirect Relationships • Directly proportional - as one variable goes up/down the other goes up/down as well. Both variables do the same thing. • Indirectly proportional - as one variable goes up/down the other goes down/up. The two variables do the opposite thing.

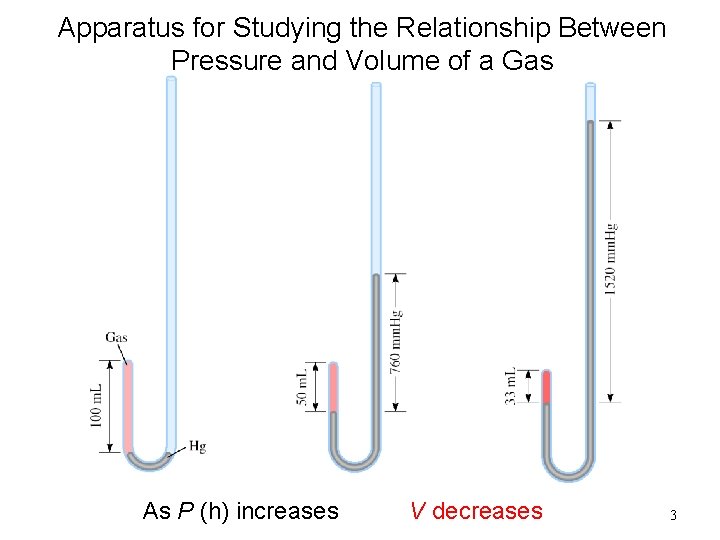
Apparatus for Studying the Relationship Between Pressure and Volume of a Gas As P (h) increases V decreases 3

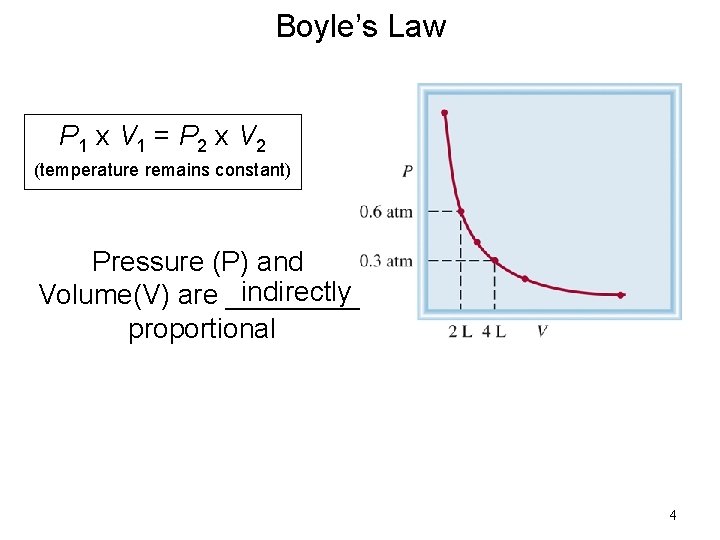
Boyle’s Law P 1 x V 1 = P 2 x V 2 (temperature remains constant) Pressure (P) and indirectly Volume(V) are _____ proportional 4

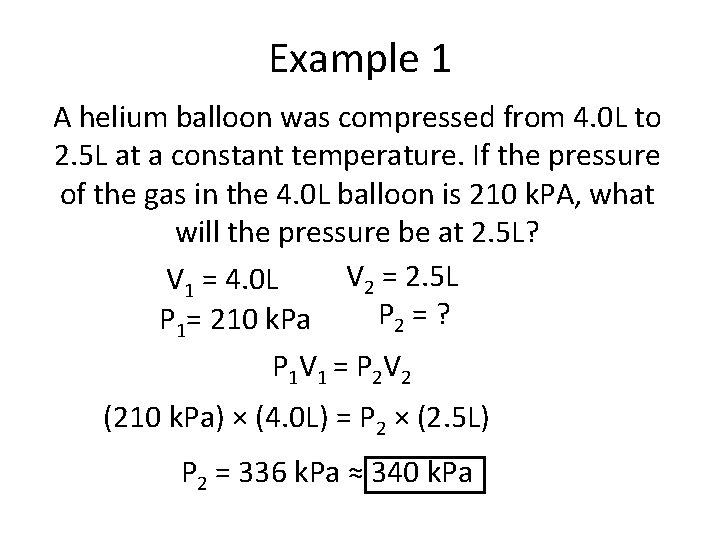
Example 1 A helium balloon was compressed from 4. 0 L to 2. 5 L at a constant temperature. If the pressure of the gas in the 4. 0 L balloon is 210 k. PA, what will the pressure be at 2. 5 L? V 2 = 2. 5 L V 1 = 4. 0 L P 2 = ? P 1= 210 k. Pa P 1 V 1 = P 2 V 2 (210 k. Pa) × (4. 0 L) = P 2 × (2. 5 L) P 2 = 336 k. Pa ≈ 340 k. Pa

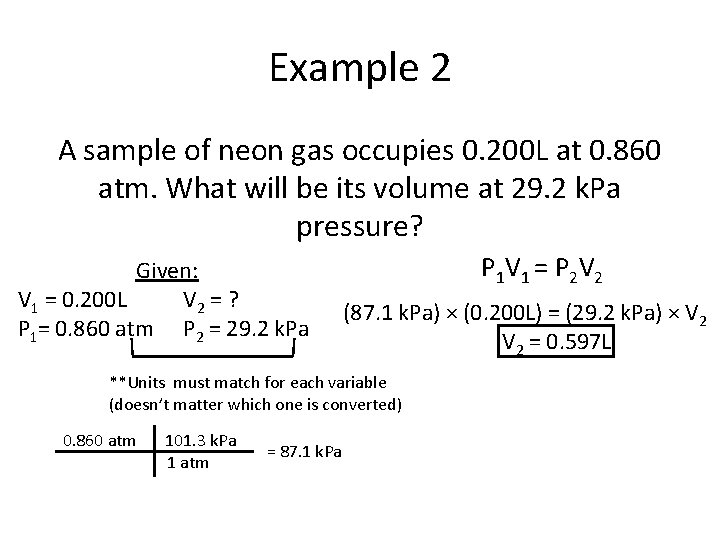
Example 2 A sample of neon gas occupies 0. 200 L at 0. 860 atm. What will be its volume at 29. 2 k. Pa pressure? Given: V 1 = 0. 200 L V 2 = ? P 1= 0. 860 atm P 2 = 29. 2 k. Pa P 1 V 1 = P 2 V 2 (87. 1 k. Pa) × (0. 200 L) = (29. 2 k. Pa) × V 2 = 0. 597 L **Units must match for each variable (doesn’t matter which one is converted) 0. 860 atm 101. 3 k. Pa 1 atm = 87. 1 k. Pa

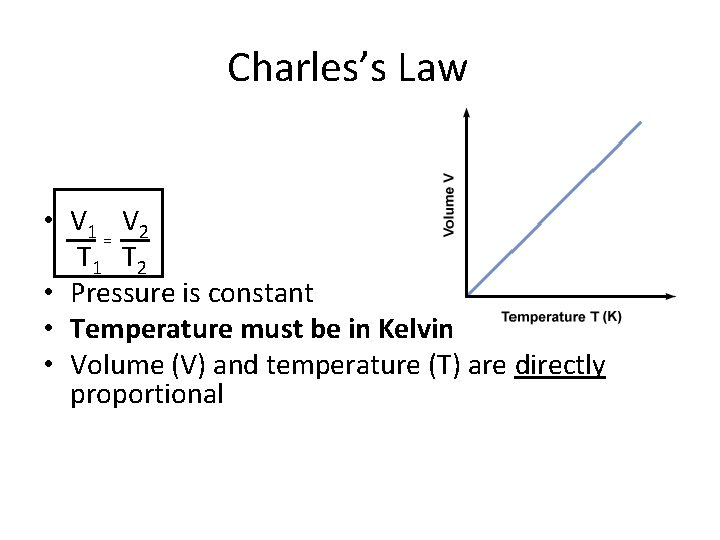
Charles’s Law • V 1 V 2 = T 1 T 2 • Pressure is constant • Temperature must be in Kelvin • Volume (V) and temperature (T) are directly proportional

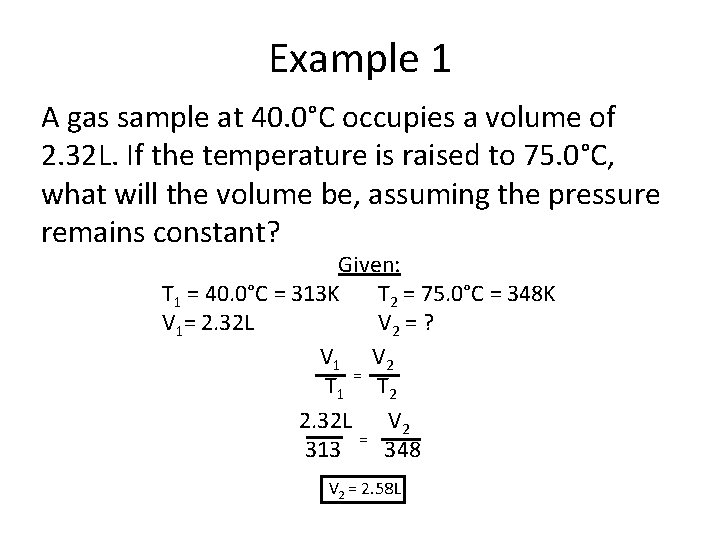
Example 1 A gas sample at 40. 0°C occupies a volume of 2. 32 L. If the temperature is raised to 75. 0°C, what will the volume be, assuming the pressure remains constant? Given: T 1 = 40. 0°C = 313 K T 2 = 75. 0°C = 348 K V 1= 2. 32 L V 2 = ? V 1 V 2 T 1 = T 2 2. 32 L V 2 313 = 348 V 2 = 2. 58 L

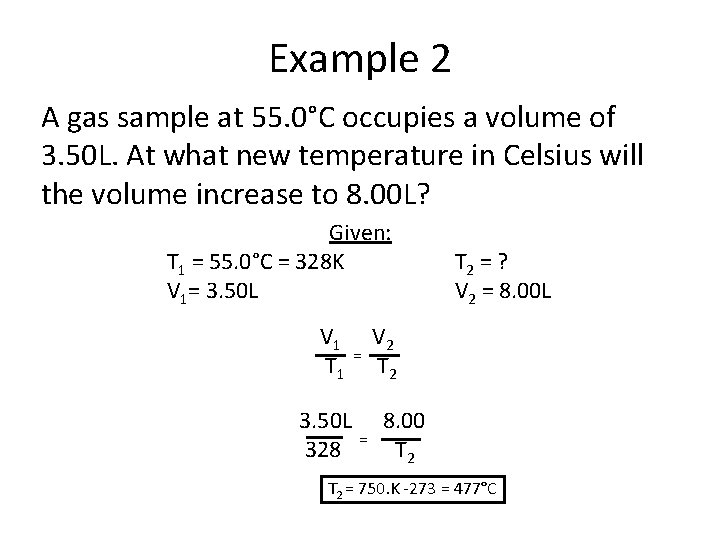
Example 2 A gas sample at 55. 0°C occupies a volume of 3. 50 L. At what new temperature in Celsius will the volume increase to 8. 00 L? Given: T 1 = 55. 0°C = 328 K V 1= 3. 50 L V 1 T 1 3. 50 L 328 = = T 2 = ? V 2 = 8. 00 L V 2 T 2 8. 00 T 2 = 750. K -273 = 477°C

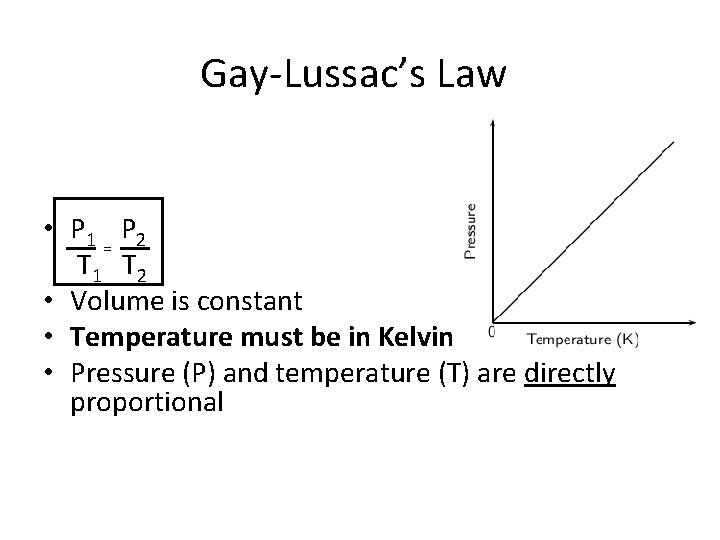
Gay-Lussac’s Law • P 1 P 2 = T 1 T 2 • Volume is constant • Temperature must be in Kelvin • Pressure (P) and temperature (T) are directly proportional

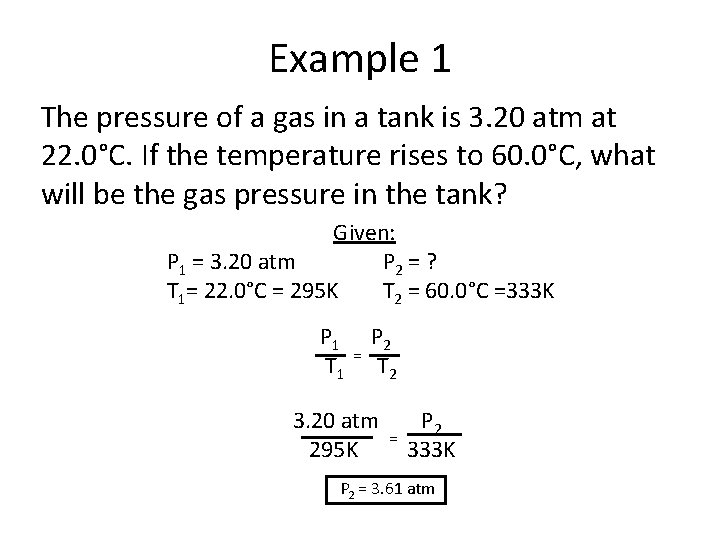
Example 1 The pressure of a gas in a tank is 3. 20 atm at 22. 0°C. If the temperature rises to 60. 0°C, what will be the gas pressure in the tank? Given: P 1 = 3. 20 atm P 2 = ? T 1= 22. 0°C = 295 K T 2 = 60. 0°C =333 K P 1 T 1 = P 2 T 2 3. 20 atm 295 K = P 2 333 K P 2 = 3. 61 atm

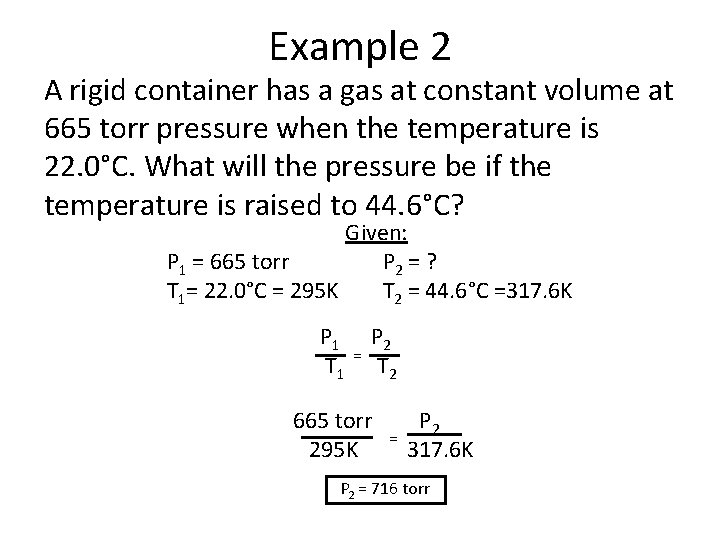
Example 2 A rigid container has a gas at constant volume at 665 torr pressure when the temperature is 22. 0°C. What will the pressure be if the temperature is raised to 44. 6°C? Given: P 1 = 665 torr P 2 = ? T 1= 22. 0°C = 295 K T 2 = 44. 6°C =317. 6 K P 1 T 1 = P 2 T 2 665 torr 295 K = P 2 317. 6 K P 2 = 716 torr