The Ideal Gas Law Ideal Gas Made of


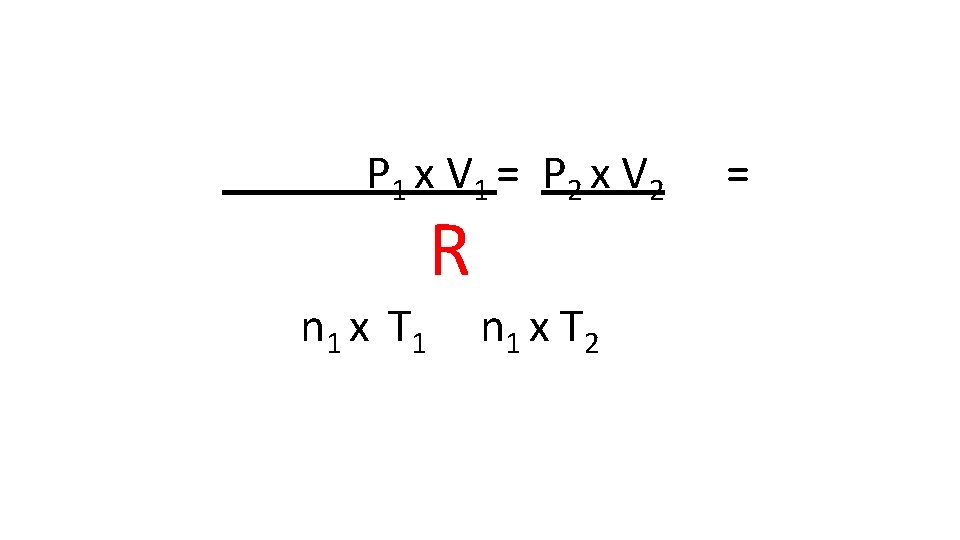









- Slides: 17

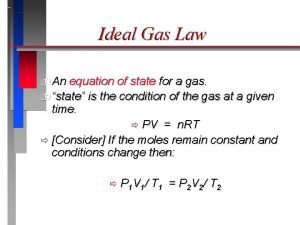
The Ideal Gas Law

Ideal Gas Made of small particles that have mass Mostly Empty Space Low density Particles are in constant motion No attractive or repulsive forces between particles Particles have no volume Collision are elastic (lose no KE) Real Gas Same Small attractive or repulsive forces between particles Particles have small volume Collision are NOT elastic (lose KE)
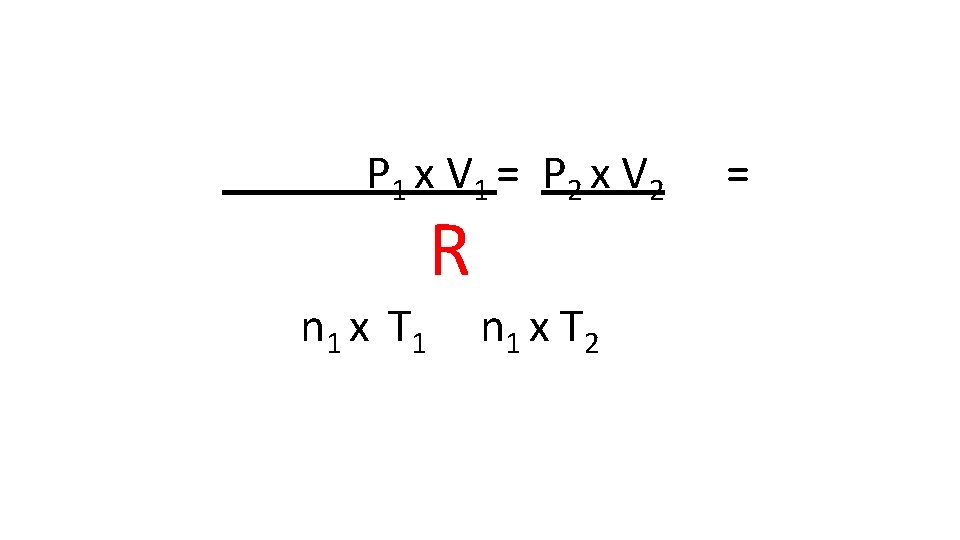
P 1 x V 1 = P 2 x V 2 n 1 x T 1 R n 1 x T 2 =


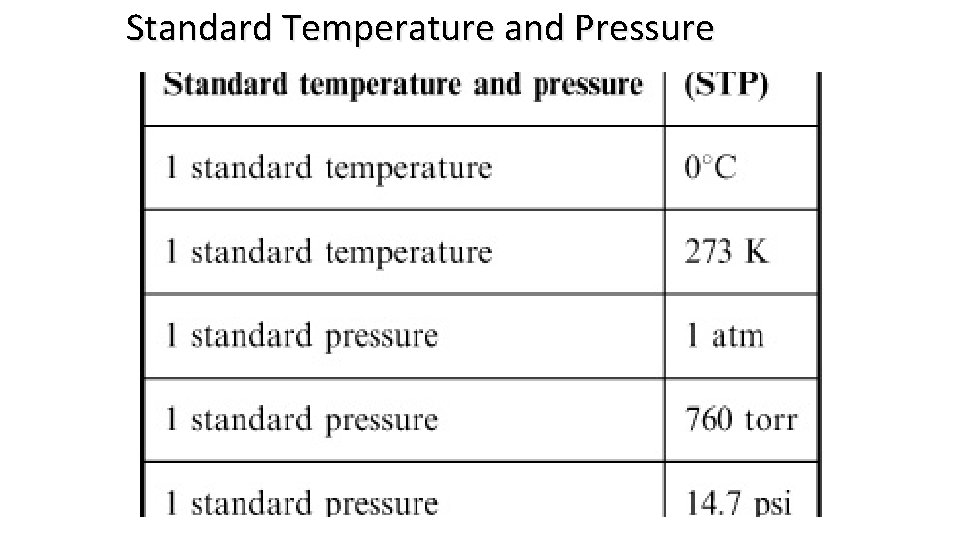
Standard Temperature and Pressure

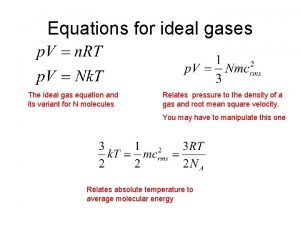
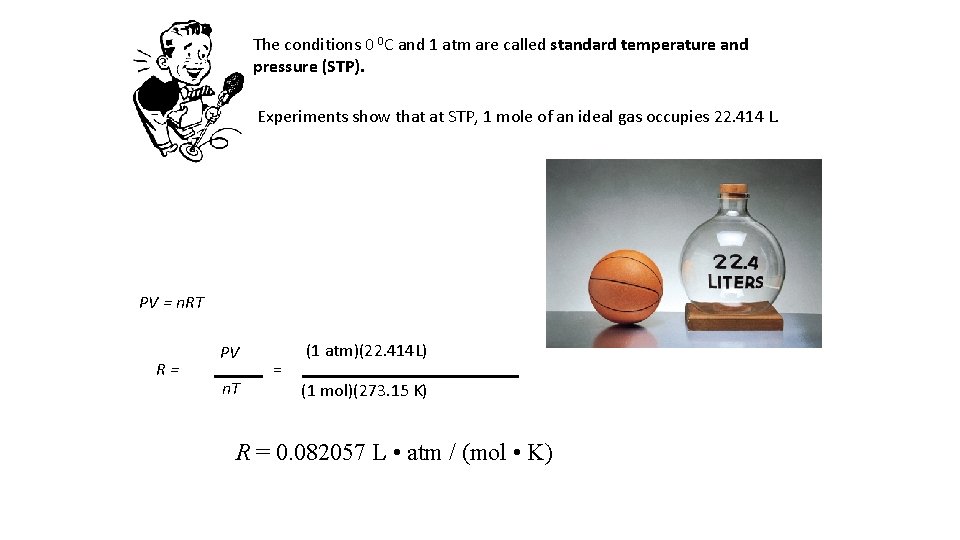
The conditions 0 0 C and 1 atm are called standard temperature and pressure (STP). Experiments show that at STP, 1 mole of an ideal gas occupies 22. 414 L. PV = n. RT R= PV n. T = (1 atm)(22. 414 L) (1 mol)(273. 15 K) R = 0. 082057 L • atm / (mol • K)

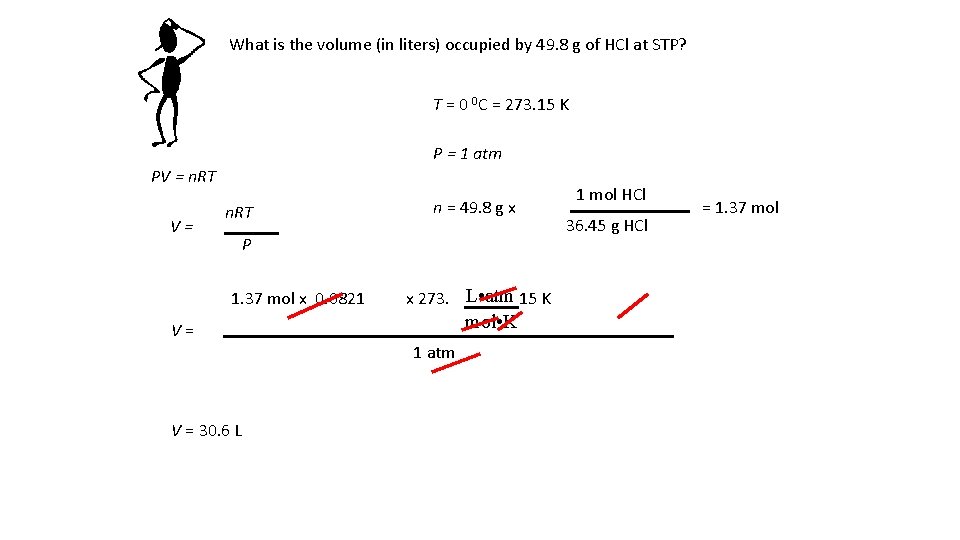
What is the volume (in liters) occupied by 49. 8 g of HCl at STP? T = 0 0 C = 273. 15 K P = 1 atm PV = n. RT V= n. RT n = 49. 8 g x P 1. 37 mol x 0. 0821 V= V = 30. 6 L x 273. L • atm 15 K mol • K 1 atm 1 mol HCl 36. 45 g HCl = 1. 37 mol

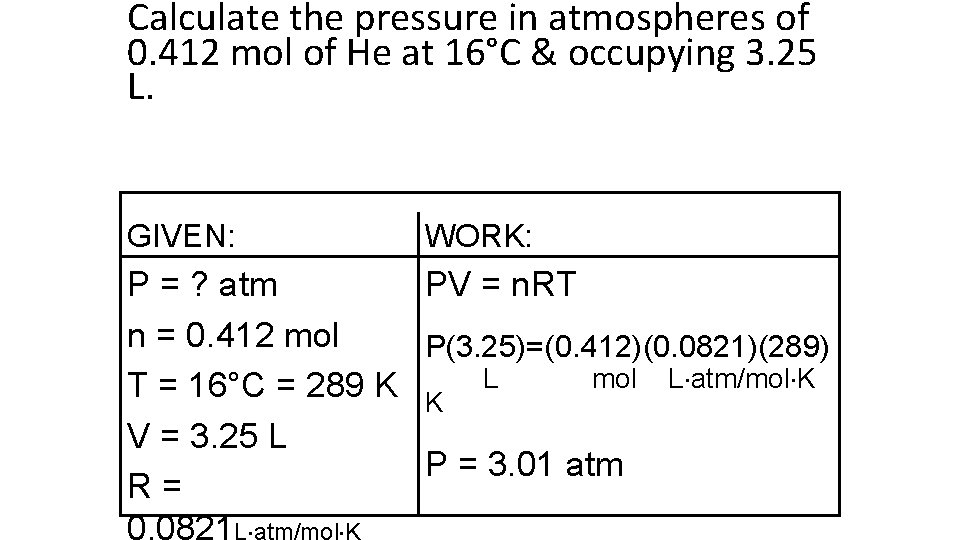
Calculate the pressure in atmospheres of 0. 412 mol of He at 16°C & occupying 3. 25 L. GIVEN: WORK: P = ? atm PV = n. RT n = 0. 412 mol P(3. 25)=(0. 412)(0. 0821)(289) mol L atm/mol K T = 16°C = 289 K K L V = 3. 25 L P = 3. 01 atm R= 0. 0821 L atm/mol K

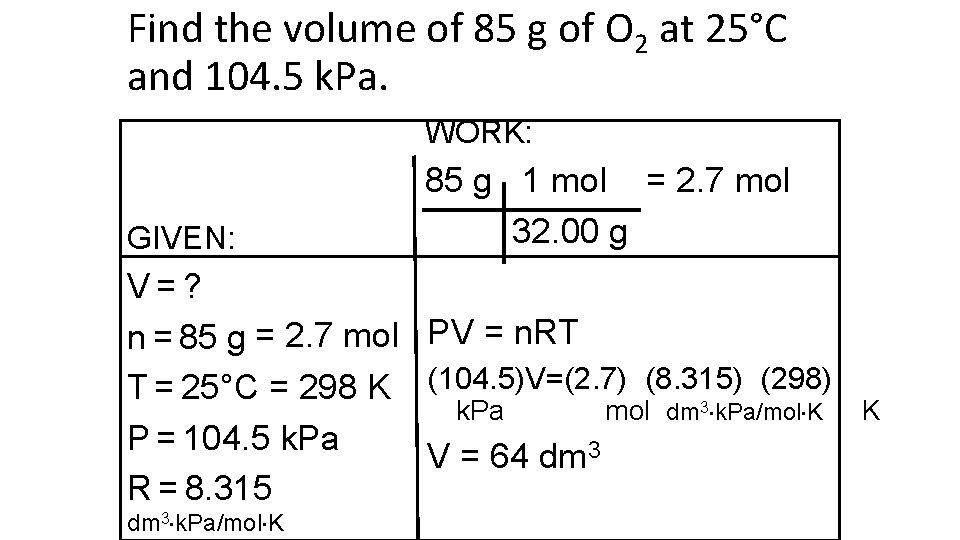
Find the volume of 85 g of O 2 at 25°C and 104. 5 k. Pa. WORK: GIVEN: 85 g 1 mol = 2. 7 mol 32. 00 g V=? n = 85 g = 2. 7 mol PV = n. RT (298) T = 25°C = 298 K (104. 5)V=(2. 7) (8. 315) k. Pa mol dm 3 k. Pa/mol K P = 104. 5 k. Pa V = 64 dm 3 R = 8. 315 dm 3 k. Pa/mol K K

Example A gas sample occupies a volume of 0. 685 L at 38°C and 0. 775 atm. What will be the temperature of the sample if it occupies 0. 125 L at 1. 25 atm?

Example A sample of nitrogen gas in a 2. 00 L container has a pressure of 1800. 6 mm. Hg at 25°C. What would be the pressure on the sample in a 1. 00 L container at 125°C?

Example The gas pressure in an aerosol can is 1. 5 atm at 25 o. C. Assuming that the gas inside obeys the ideal-gas equation, what would the pressure be if the can were heated to 450 o. C?

What volume does 48. 0 g of methane, CH 4, occupy at 140. o. C under a pressure of 1280 torr?

A 250. m. L flask contains a mixture of nitrogen, oxygen, and helium at a temperature of 27 o. C and a pressure of 0. 850 atm. How many moles of gas are present?

What pressure in kilopascals is exerted by 0. 480 g of carbon dioxide in a 1. 00 L flask at 100 o. C?

What is the volume of a 15. 0 -g sample of neon gas at 130. 0 k. Pa and 39°C?

At what temperature will 2. 85 moles of hydrogen gas occupy a volume of 0. 58 L at 1. 00 atm?
 Ideal gas vs perfect gas
Ideal gas vs perfect gas An ideal gas is an imaginary gas
An ideal gas is an imaginary gas Differences between ideal gas and real gas
Differences between ideal gas and real gas Ideal gas vs perfect gas
Ideal gas vs perfect gas Difference between ideal gas and real gas
Difference between ideal gas and real gas Unit of pressure
Unit of pressure What's an ideal gas
What's an ideal gas Avogadro's law units
Avogadro's law units Which equation agrees with the ideal gas law?
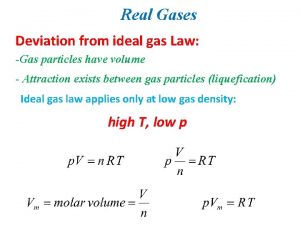
Which equation agrees with the ideal gas law? Deviation from ideal gas
Deviation from ideal gas Ideal gas law examples
Ideal gas law examples Deviations from the ideal gas law
Deviations from the ideal gas law Ideal gas law
Ideal gas law Density gas equation
Density gas equation State ideal gas equation
State ideal gas equation R constant chemistry
R constant chemistry Stp vs satp
Stp vs satp Ideal gas law formula
Ideal gas law formula