Gas Laws Chapter 11 Kinetic Molecular Theory KMT


















- Slides: 26

Gas Laws Chapter 11

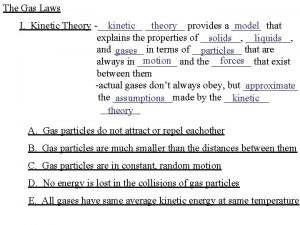
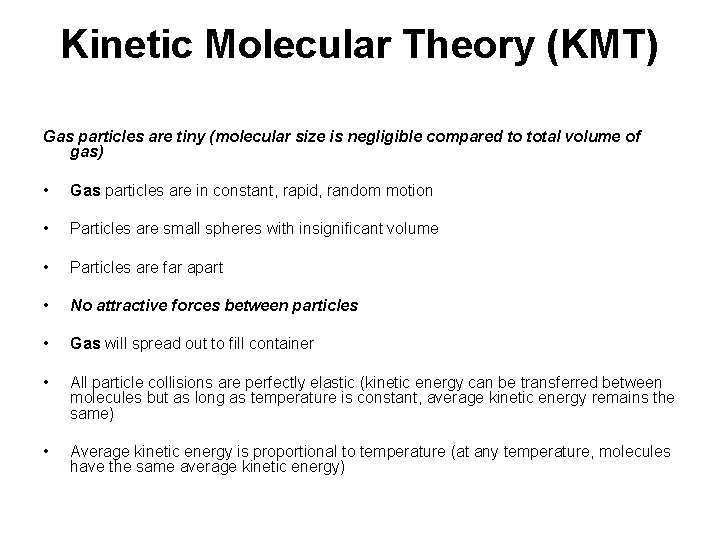
Kinetic Molecular Theory (KMT) Gas particles are tiny (molecular size is negligible compared to total volume of gas) • Gas particles are in constant, rapid, random motion • Particles are small spheres with insignificant volume • Particles are far apart • No attractive forces between particles • Gas will spread out to fill container • All particle collisions are perfectly elastic (kinetic energy can be transferred between molecules but as long as temperature is constant, average kinetic energy remains the same) • Average kinetic energy is proportional to temperature (at any temperature, molecules have the same average kinetic energy)

Pressure • Pressure = force distributed over an area • Pressure results when particles collide • No particles = no collisions, no pressure (a vacuum) • SI unit of pressure = pascal (Pa) (1 Newton/square meter) • Other pressure units: atmosphere (atm), millimeters of mercury (mm. Hg), torricelli (torr), bar • To convert: 1 atm = 760 mm. Hg = 101. 3 k. Pa = 760 torr • Also: 1000 Pa = 1 k. Pa, 1 bar = 100000 Pa • Manometer = instrument used to measure pressure based on the height of a mercury column that the gas pressure can support • Barometer = device used to measure atmospheric pressure

Temperature • Absolute temperature (temperature in Kelvin) is directly proportional to average kinetic energy of particles • http: //www. uwsp. edu/physastr/kmenning/flash/AF_2112. swf • • • Recall Temp in Kelvin = T in Celsius + 273 Temp in Celsius = T in Kelvin -273 • Absolute zero (0 Kelvin, -273 Celsius) = essentially no kinetic energy, no particle movement • Standard Temperature and Pressure • For many gas calculations “standard temperature and pressure” (STP) conditions are used as common reference conditions. Standard temperature and pressure is 1 atm (= 760 mm. Hg = 101. 3 k. Pa etc. ) and 273 K (0 degrees Celsius).

Following Links gives practice problems Boyles Law Charles Law Combined Gas Law Ideal Gas Law

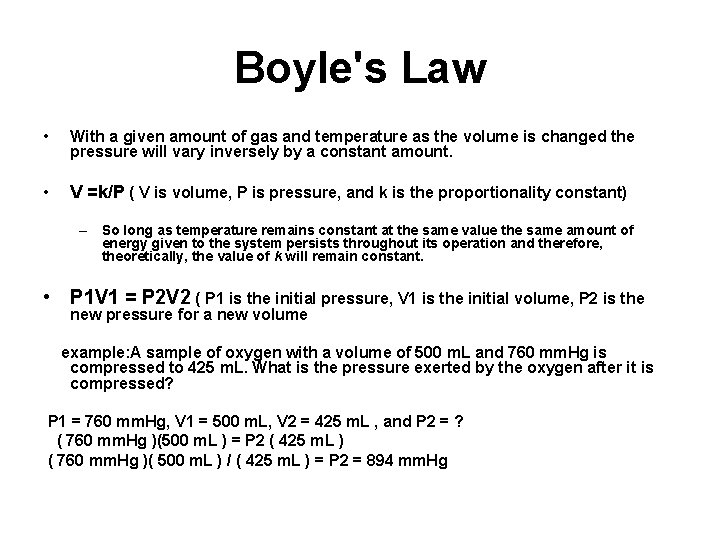
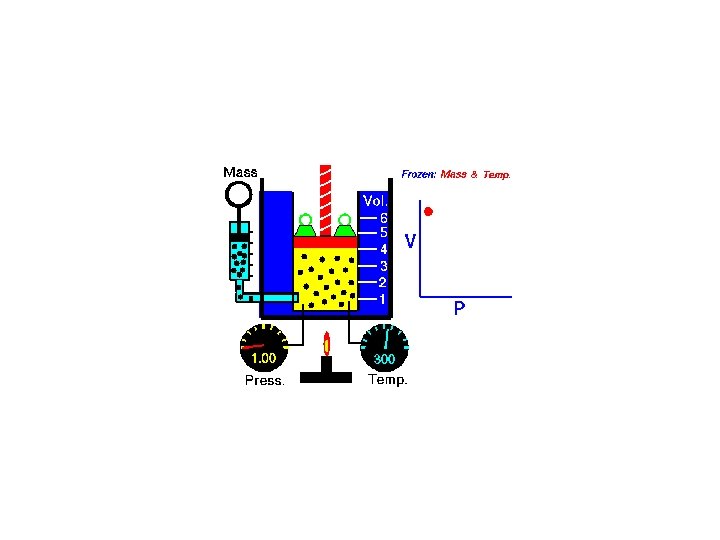
Boyle's Law • With a given amount of gas and temperature as the volume is changed the pressure will vary inversely by a constant amount. • V =k/P ( V is volume, P is pressure, and k is the proportionality constant) – So long as temperature remains constant at the same value the same amount of energy given to the system persists throughout its operation and therefore, theoretically, the value of k will remain constant. • P 1 V 1 = P 2 V 2 ( P 1 is the initial pressure, V 1 is the initial volume, P 2 is the new pressure for a new volume example: A sample of oxygen with a volume of 500 m. L and 760 mm. Hg is compressed to 425 m. L. What is the pressure exerted by the oxygen after it is compressed? P 1 = 760 mm. Hg, V 1 = 500 m. L, V 2 = 425 m. L , and P 2 = ? ( 760 mm. Hg )(500 m. L ) = P 2 ( 425 m. L ) ( 760 mm. Hg )( 500 m. L ) / ( 425 m. L ) = P 2 = 894 mm. Hg


Samples • 1. If some neon gas at 121 k. Pa were allowed to expand from 3. 7 dm 3 to 6. 0 dm 3 without changing the temperature, what pressure would the neon gas exert under these new conditions? • 2. A quantity of gas under a pressure of 1. 78 atm has a volume of 550 cm 3. The pressure is increased to 2. 50 atm, while the pressure remains constant. What is the new volume? • 3. Under a pressure of 172 k. Pa, a gas has a volume of 564 cm 3. The pressure is decreased, without changing the temperature, until the volume of the gas is equal to 8. 00 x 102 cm 3. What is the new pressure?

• • Answers: 1) 75 k. Pa 2) 390 cm 3 3) 121 k. Pa

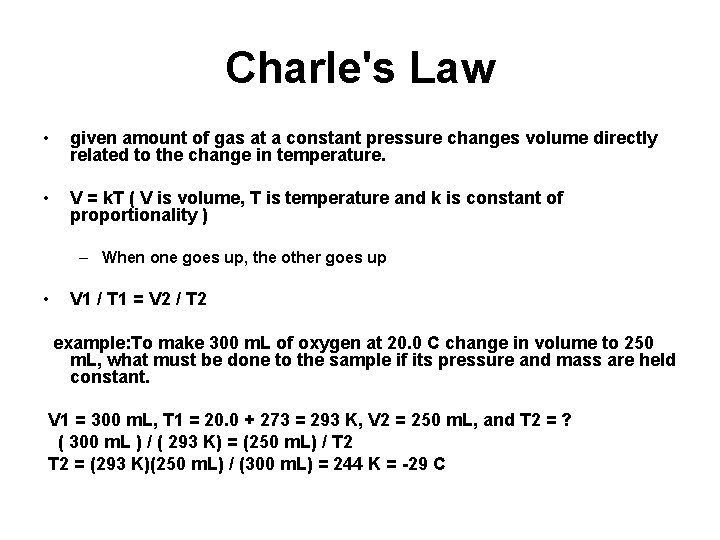
Charle's Law • given amount of gas at a constant pressure changes volume directly related to the change in temperature. • V = k. T ( V is volume, T is temperature and k is constant of proportionality ) – When one goes up, the other goes up • V 1 / T 1 = V 2 / T 2 example: To make 300 m. L of oxygen at 20. 0 C change in volume to 250 m. L, what must be done to the sample if its pressure and mass are held constant. V 1 = 300 m. L, T 1 = 20. 0 + 273 = 293 K, V 2 = 250 m. L, and T 2 = ? ( 300 m. L ) / ( 293 K) = (250 m. L) / T 2 = (293 K)(250 m. L) / (300 m. L) = 244 K = -29 C


1. What volume will a sample of hydrogen occupy at 28. 0 o. C if the gas occupies a volume of 2. 23 dm 3 at a temperature of 0. 0 o. C? Assume that the pressure remains constant. (remember to change to Kelvin). 2. If a gas occupies 733 cm 3 at 10. 0 o. C, at what temperature will it occupy 950 cm 3? Assume that pressure remains constant. 3. A gas occupies 560 cm 3 at 285 K. To what temperature must the gas be lowered to, if it is to occupy 25. 0 cm 3? Assume a constant pressure.

Answers: 1) 2. 46 dm 3 2) 370 K 3) 13 K

Gay-Lussac's Law • The pressure of given amount of gas held at constant volume is directly proportional to the Kelvin temperature. • P = k. T ( P is pressure, T is temperature, and k is constant of proportionality ) • P 1 / T 1 = P 2 / T 2 example: A sample of Nitrogen gas contained in a 50 L rigid container has a pressure of 101 k. Pa at 25 C. If the container is heated to 150 C what is the pressure in the container? P 1 = 101 k. Pa, T 1 = 25 + 273 = 298 K, T 2 = 150 + 273 = 423 K, and P 2 = ? (101 k. Pa) / (298 K) = P 2 / (423 K) (101 k. Pa)(423 K) / (298 K) = P 2 = 143 k. Pa

Combined Gas Law • The volume of a given amount of gas is proportional to the ratio of its Kelvin temperature and its pressure. • PV = k. T • P 1 V 1/T 1 = P 2 V 2/T 2 – Memorize just the combined, and you can reduce it to any of the other three by cancelling out the variable you don’t need! – Or, if you need to use all variables because more than one condition changes, keep them all… example: What will be the final pressure of a sample of nitrogen with a volume of 950 L at 745 torr and 25. 0 C if it is heated to 60. 0 C and given a final volume of 1150 L? P 1 = 745 torr, V 1 =950 L, T 1 = 25. 0 + 273. 2 = 298. 2 K, V 2 = 1150 L , T 2 = 60. 0 + 273. 2 = 333. 2 K, and P 2 = ? (745 torr)(950 L) / (298. 2 K) = P 2 (1150 L) / (333. 2 K) (745 torr)(950 L)(333. 2 K) / [(298. 2 K)(1150 L)] = P 2 = 688 torr

Avogadro's Principle • When measured at the same temperature and pressure, equal volume of gases contain equal number of moles. • 1 mol of gas at STP occupies approximately 22. 4 Liters.

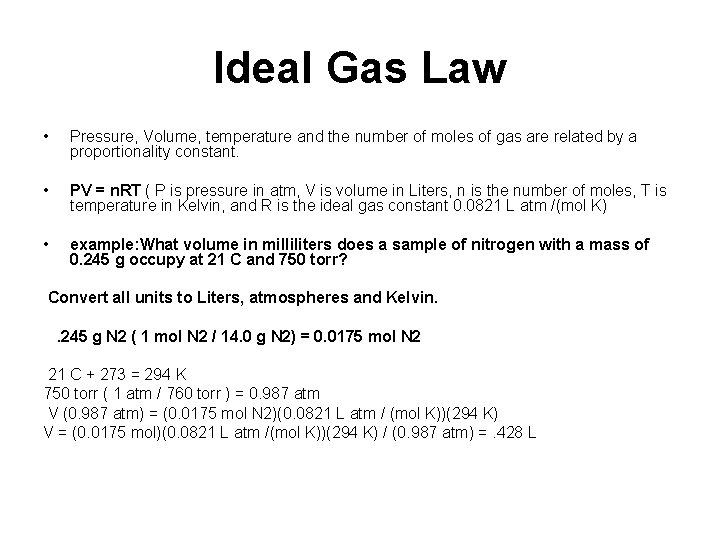
Ideal Gas Law • Pressure, Volume, temperature and the number of moles of gas are related by a proportionality constant. • PV = n. RT ( P is pressure in atm, V is volume in Liters, n is the number of moles, T is temperature in Kelvin, and R is the ideal gas constant 0. 0821 L atm /(mol K) • example: What volume in milliliters does a sample of nitrogen with a mass of 0. 245 g occupy at 21 C and 750 torr? Convert all units to Liters, atmospheres and Kelvin. . 245 g N 2 ( 1 mol N 2 / 14. 0 g N 2) = 0. 0175 mol N 2 21 C + 273 = 294 K 750 torr ( 1 atm / 760 torr ) = 0. 987 atm V (0. 987 atm) = (0. 0175 mol N 2)(0. 0821 L atm / (mol K))(294 K) V = (0. 0175 mol)(0. 0821 L atm /(mol K))(294 K) / (0. 987 atm) =. 428 L

Dalton's Law of Partial Pressures • The total pressure of a mixture of nonreacting gases is the sum of their individual partial pressure. • Ptotal = Pa + Pb + Pc. . . • example: How many grams of oxygen are present at 25 C in a 5. 00 L tank of oxygen-enriched air under a total pressure of 30. 0 atm when the only other gas is nitrogen at a partial pressure of 15. 0 atm? Ptotal = 30. 0 atm, PN 2 = 15. 0 atm, PO 2 = ? 30. 0 atm = 15. 0 atm + PO 2, PO 2 = 15. 0 atm (15. 0 atm)(5. 00 L) = n (0. 0821 L atm / (mol K))(298 K), solve for n. n = 3. 07 mol O 2 ( 32. 0 g O 2 / 1 mol O 2) = 98. 2 g O 2

• Daltons Law and Mole fractions

Vapor Pressure • The amount of pressure exerted above a liquid by particles escaping from the liquid into a gas phase. Vapor pressure of any substance is dependent on the temperature. The higher the temperature the greater the amount of energy in the molecules the easier it is for the particles to escape.

Gas Law from Virtual Chemistry

Real Gas • ideal gas real gas obey PV=n. RT: always only at very low pressures molecularvolume: zero small, but nonzero molecular attractions: zero small molecular repulsions: zero small

• distance between molecules is related to gas concentration: n/V = P/RT • high gas concentration = closer molecules = stronger intermolecular interactions = deviations from ideal behavior – repulsions make pressure higher than expected by decreasing free volume – attractions make pressure lower than expected by braking molecular collisions – tug-of-war between these two effects • repulsions win at very high pressure • attractions win at moderate pressure • neither attractions nor repulsions are important at low pressure

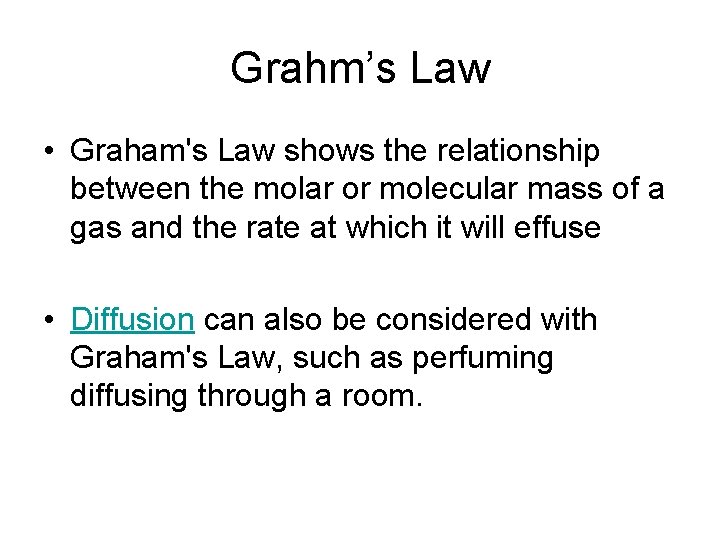
Grahm’s Law • Graham's Law shows the relationship between the molar or molecular mass of a gas and the rate at which it will effuse • Diffusion can also be considered with Graham's Law, such as perfuming diffusing through a room.

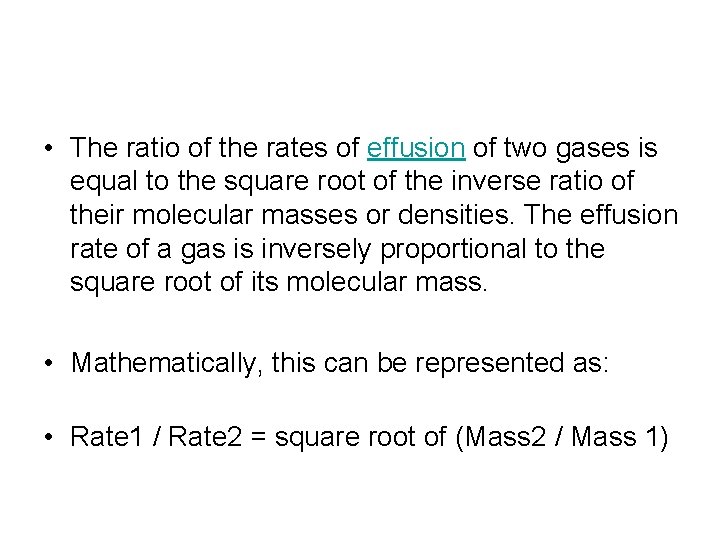
• The ratio of the rates of effusion of two gases is equal to the square root of the inverse ratio of their molecular masses or densities. The effusion rate of a gas is inversely proportional to the square root of its molecular mass. • Mathematically, this can be represented as: • Rate 1 / Rate 2 = square root of (Mass 2 / Mass 1)

• If given densities, must convert to molar mass by way of 22. 4 L/mol
 Kmt gas laws
Kmt gas laws Kinetic molecular theory of solids
Kinetic molecular theory of solids Kinetic theory for ideal gases
Kinetic theory for ideal gases The kinetic molecular theory
The kinetic molecular theory Adhesive force
Adhesive force Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Kinetic molecular theory
Kinetic molecular theory Kinetic theory def
Kinetic theory def Timeline of kinetic molecular theory
Timeline of kinetic molecular theory Charles law in terms of kinetic molecular theory
Charles law in terms of kinetic molecular theory Kinetic theory postulates
Kinetic theory postulates Kinetic molecular theory
Kinetic molecular theory Kinetic molecular theory
Kinetic molecular theory Pv=1/3nmc^2
Pv=1/3nmc^2 Kinetic molecular theory
Kinetic molecular theory Tenets of kinetic molecular theory
Tenets of kinetic molecular theory Principles of kmt
Principles of kmt Postulate 2 states that gas particles are:
Postulate 2 states that gas particles are: Kmt
Kmt Kmt
Kmt Kmt sct
Kmt sct Charles' law worksheet answers
Charles' law worksheet answers Vbt and mot theory
Vbt and mot theory Valence bond theory shapes
Valence bond theory shapes Valence bond theory and molecular orbital theory
Valence bond theory and molecular orbital theory Theories of covalent bonding
Theories of covalent bonding Valence bond theory hybridization
Valence bond theory hybridization