Kinetic Molecular Theory Kinetic Molecular Theory is the










- Slides: 14

Kinetic Molecular Theory

Kinetic Molecular Theory – is the idea that matter is made from moving invisible particles. The following are the principals of Kinetic Molecular Theory: All matter is made up of tiny particles Different substances have different particles The particles are in constant motion The more energy the particles have, the faster they move. The attraction between particles decreases with an increase in distance.

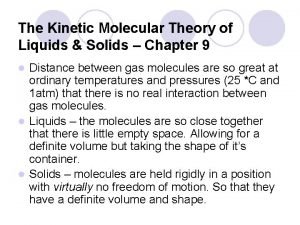
Solids Distance Particles are close together Type of Motion Particles can only vibrate in their place in the structure Attractive Forces High, decreasing as vibration gets higher Energy Increasing energy causes an increase of vibration

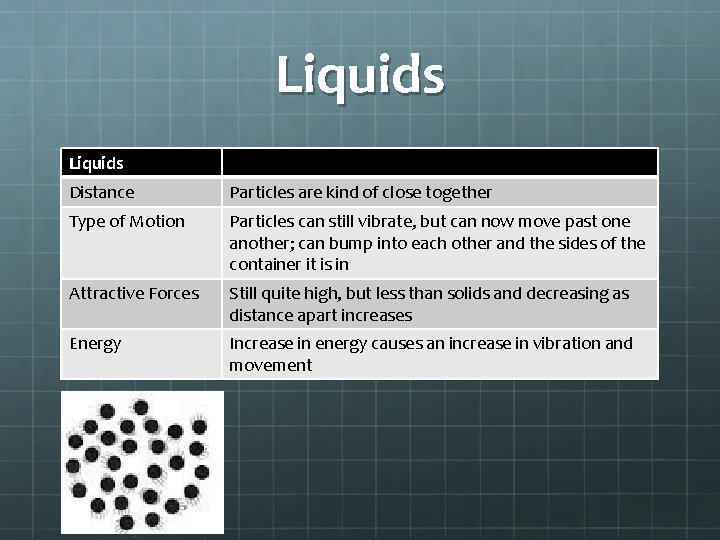
Liquids Distance Particles are kind of close together Type of Motion Particles can still vibrate, but can now move past one another; can bump into each other and the sides of the container it is in Attractive Forces Still quite high, but less than solids and decreasing as distance apart increases Energy Increase in energy causes an increase in vibration and movement

Gas Distance Particles are very far apart Type of motion Particles vibrate, rotate, move past each other, and bump into each other in a very rapid straight line of motion Attractive forces No attractive forces; particles are too far apart and are moving to fast. Energy Increase in energy causes an increase in pressure due to the increase in the speed and number of particles hitting the sides of the container.

Change of State The change of state only happens when you add or remove energy. What we experience as heat, molecules experience as vibration. An increase in movement of particles results in the melting or sublimation of a solid, and evaporation of a liquid. The reduction in movement of particles results in the condensation or deposition in gases and solidification in liquids.

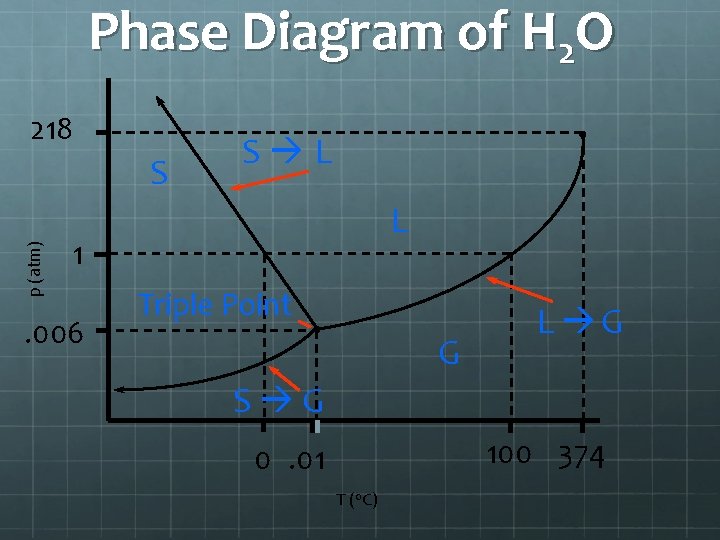
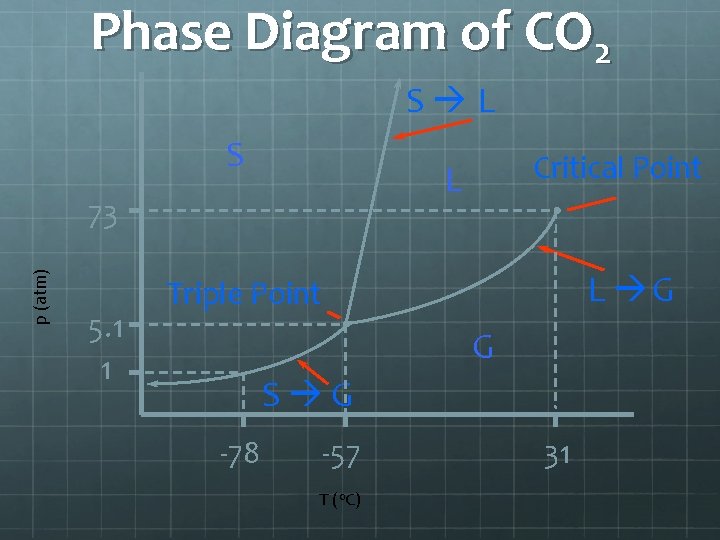
A word on sublimation and deposition Sublimation, and deposition, is the ability to turn a solid into gas or a gas into a solid without passing through the liquid stage. In order for this to occur you need to have an object reach a specific temperature and a specific pressure. The point in which sublimation and deposition is called the triple point. The point on a pressure vs temperature graph where solid, liquid and gas meet.

Triple point of Water and Carbon Dioxide Water’s triple point – where a solid piece of water, liquid and gas can all co-exist - is at 273. 16 K (0. 01º C) and a pressure of 611. 73 pascals (. 006 atm). It is at this point where water in a solid form and has a gas coming off of it. Dry ice’s (Carbon dioxide’s) triple point is 518 k. Pa (kilopascals) and -56. 6ºC or 216. 55 Kelvin.

Phase Diagram of H 2 O 218 p (atm) S S L L 1 . 006 Triple Point G L G S G 100 374 0. 01 T (o. C)

Phase Diagram of CO 2 S L S p (atm) 73 5. 1 1 Critical Point L L G Triple Point G S G -78 -57 T (o. C) 31

Water Lets take water for a moment: When it is in a solid form and we add energy (heat) we notice that the molecules vibrate a lot and turns into a liquid. When it is in a liquid form and we add energy (heat) we notice that the molecules vibration increases and turns into a gas. But if we cool the molecule at any stage, we slow down the vibration of the molecules and can reverse the process.

Explaining Dissolving The kinetic molecular theory perfectly describes how compounds, like sugar, dissolve in water. When water is in a liquid state, the molecules are vibrating and have space between them for sugar to enter into the water and dissolve. If you increase the heat of the water, the vibration of the water molecule increases, thus more space is created between the water molecules. Thus sugar has more space to enter in between the water molecules and thus the sugar will dissolve a lot faster.

Explaining Density Problem, why is it that the density of water in solid form has a different density when it is in liquid form and a different density when it is in a gas form? Answer, the kinetic molecular theory. When you add energy to a particle and increase the vibration of the particles in the compound expands and thus the volume changes. If the volume increases and the mass stays the same, the density decreases and viola, we now know exactly why ice floats in water.

Finally…. Ever notice on sidewalks and on train tracks there are gaps between the concrete sidewalk and concrete road? Well kinetic molecular theory explains why we need this. If a sidewalk was one solid strip without any gaps, during cooler months the sidewalk contracts and during warmer months the sidewalk expands. This constant motion of contraction and expansion leads to the sidewalk cracking or buckling. If you put in gap when laying the concrete, you mitigate and minimize the amount of cracks that will be created.
 Chapter 14 solids liquids and gases
Chapter 14 solids liquids and gases Kinetic molecular theory of gases
Kinetic molecular theory of gases The kinetic molecular theory
The kinetic molecular theory Adhesive force
Adhesive force Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Kinetic molecular theory
Kinetic molecular theory Kinetic molecular theory def
Kinetic molecular theory def Timeline of kinetic molecular theory
Timeline of kinetic molecular theory Charles law in terms of kinetic molecular theory
Charles law in terms of kinetic molecular theory Postulate of kinetic theory
Postulate of kinetic theory Ideal gas law examples
Ideal gas law examples Postulates of kinetic theory of gases
Postulates of kinetic theory of gases Kinetic molecular theory formula
Kinetic molecular theory formula Kinetic molecular theory
Kinetic molecular theory Tenets of kinetic molecular theory
Tenets of kinetic molecular theory