States of Matter Common States of Matter Gases












- Slides: 29

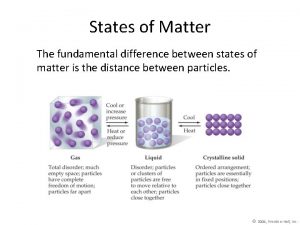
States of Matter

Common States of Matter Gases What do you know about gases? Liquids What do you know about liquids? Solids What do you know about solids? States of matter phet

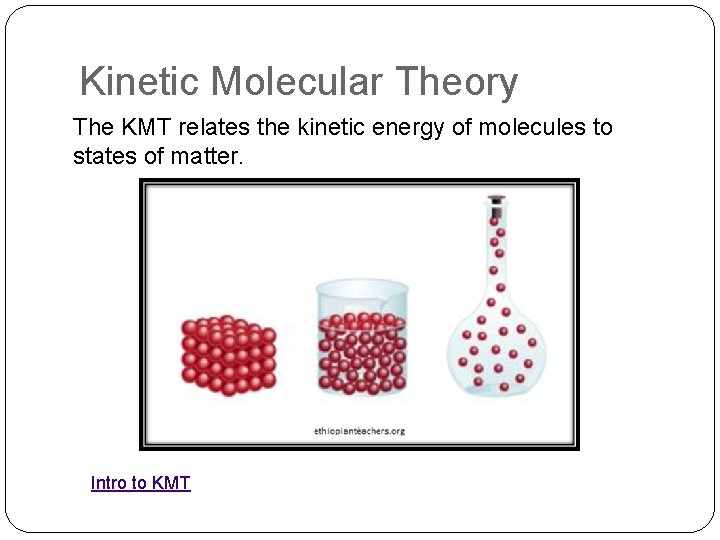
Kinetic Molecular Theory The KMT relates the kinetic energy of molecules to states of matter. Intro to KMT

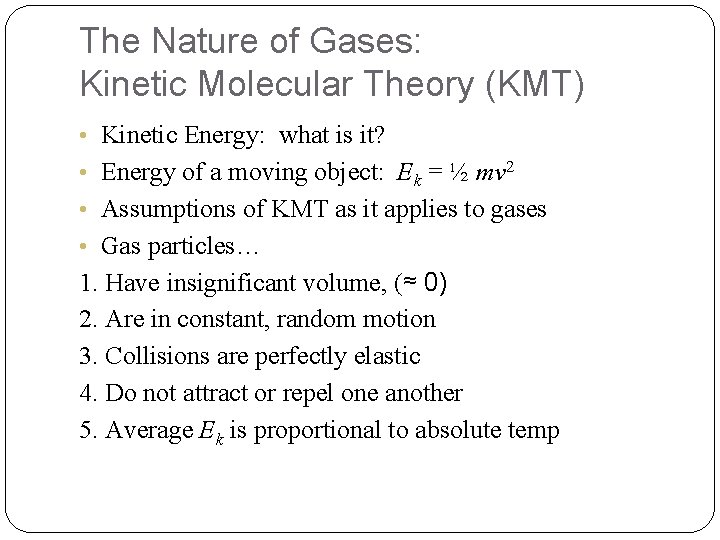
The Nature of Gases: Kinetic Molecular Theory (KMT) • Kinetic Energy: what is it? • Energy of a moving object: Ek = ½ mv 2 • Assumptions of KMT as it applies to gases • Gas particles… 1. Have insignificant volume, (≈ 0) 2. Are in constant, random motion 3. Collisions are perfectly elastic 4. Do not attract or repel one another 5. Average Ek is proportional to absolute temp

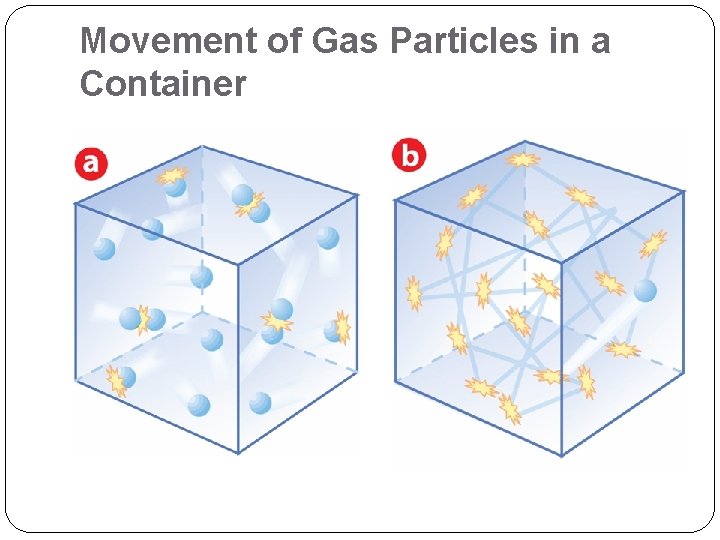
Movement of Gas Particles in a Container

Gas Pressure • Pressure = Force/Area: • P = F/A • Gas pressure is the result of collision of gas particles with an object. • Why is there no pressure in a vacuum? • It is the sum of the force of collisions per unit area

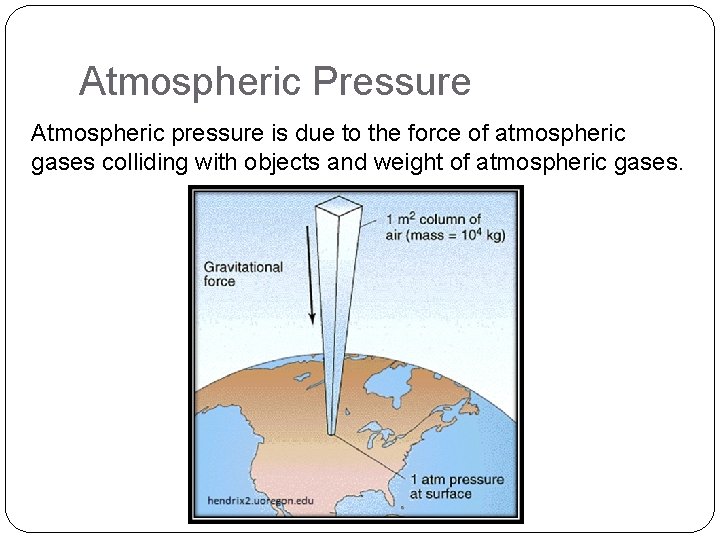
Atmospheric Pressure Atmospheric pressure is due to the force of atmospheric gases colliding with objects and weight of atmospheric gases.

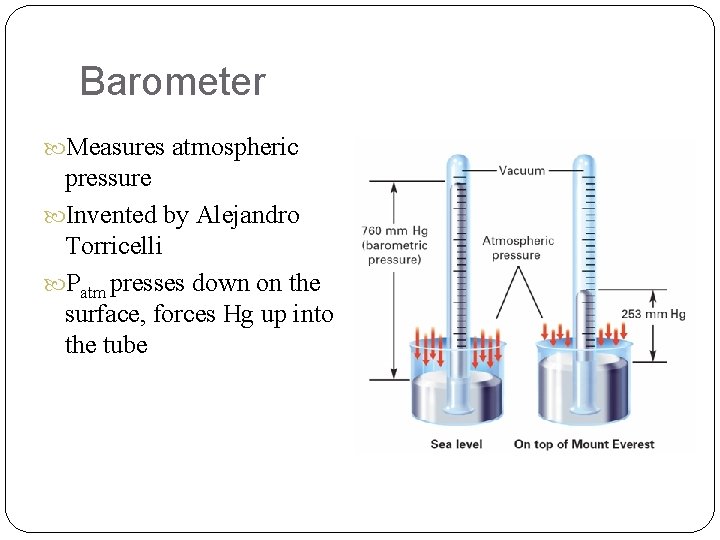
Barometer Measures atmospheric pressure Invented by Alejandro Torricelli Patm presses down on the surface, forces Hg up into the tube

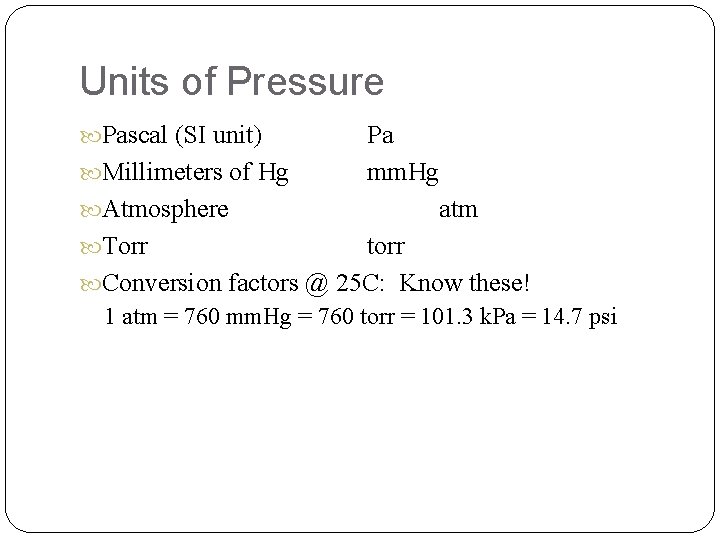
Units of Pressure Pascal (SI unit) Pa Millimeters of Hg mm. Hg Atmosphere atm Torr torr Conversion factors @ 25 C: Know these! 1 atm = 760 mm. Hg = 760 torr = 101. 3 k. Pa = 14. 7 psi

Converting Between Units of Pressure Convert a pressure of 385 mm. Hg to kilopascals (k. Pa) How would you do it? 51. 3 k. Pa

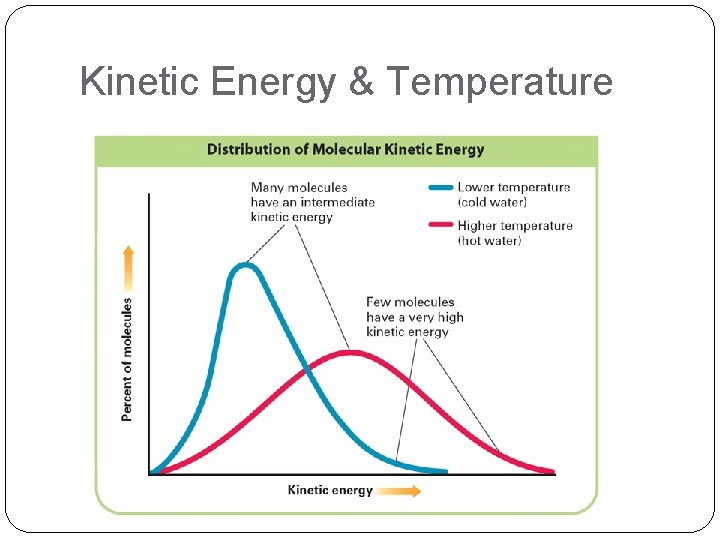
Kinetic Energy & Temperature

13. 2 The Nature of Liquids Both liquids and gases are fluids, i. e. they can flow

13. 2 The Nature of Liquids Key difference from gases: Molecules are close enough to have intermolecular forces of attraction This is why liquids have a definite volume But not close enough to fix them in place This is why molecules of liquids can move past one another (flow) Condensed matter: Liquids and solids are known as condensed phases of matter

Evaporation

Evaporation Vaporization: the conversion of a liquid to a gas or vapor Evaporation: vaporization occurring at the surface of a liquid During evaporation, molecules of liquid with sufficient KE “escape” in to the vapor phase In a closed container, some molecules that escaped reenter into the liquid phase (condense) Eventually and equilibrium is reached where… The rate of evaporation equals the rate of condensation What would happen to the rate of evaporation when a liquid is heated? Why?

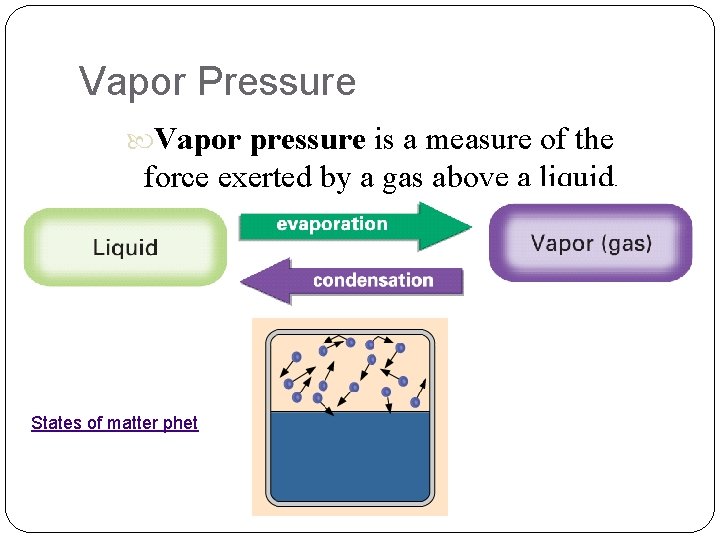
13. 2 Vapor Pressure Vapor pressure is a measure of the force exerted by a gas above a liquid. States of matter phet

Vapor Pressure In a closed container, as molecules escape into the vapor phase, pressure builds This is vapor pressure At a certain pressure, the rate of vaporization equals the rate of condensation This is an example of “dynamic equilibrium” Then vapor pressure is constant Vapor Pressure Simulation

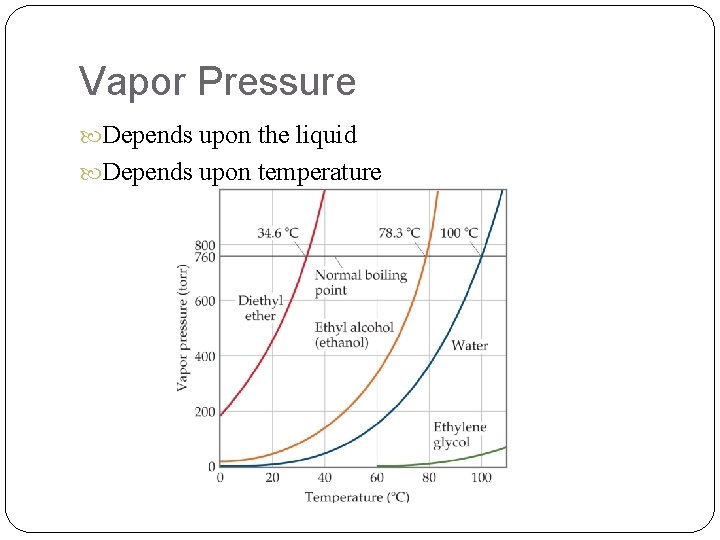
Vapor Pressure Depends upon the liquid Depends upon temperature

Vapor Pressure Depends upon the liquid Depends upon temperature

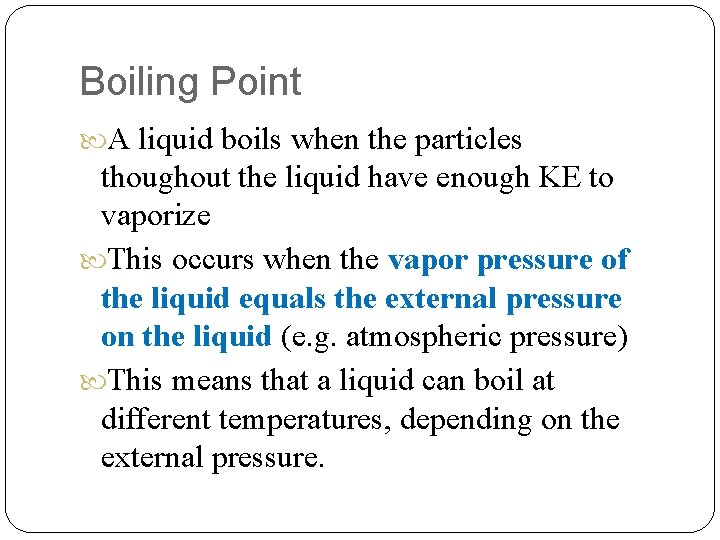
Boiling Point A liquid boils when the particles thoughout the liquid have enough KE to vaporize This occurs when the vapor pressure of the liquid equals the external pressure on the liquid (e. g. atmospheric pressure) This means that a liquid can boil at different temperatures, depending on the external pressure.

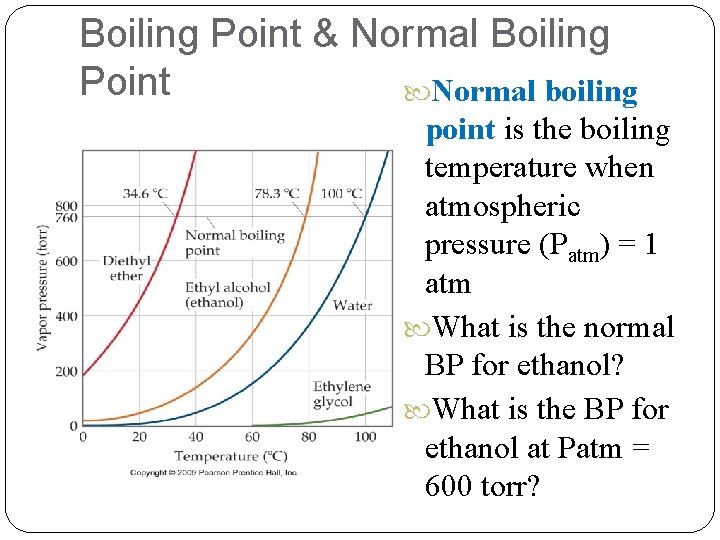
Boiling Point & Normal Boiling Point Normal boiling point is the boiling temperature when atmospheric pressure (Patm) = 1 atm What is the normal BP for ethanol? What is the BP for ethanol at Patm = 600 torr?

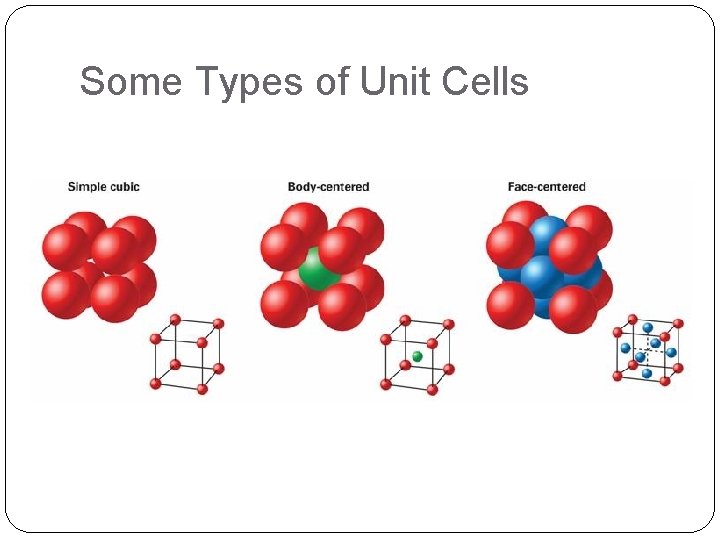
13. 3 Solids Crystal Structure Simple cubic Body centered cubic Face centered cubic Allotropes Amorphous solids Glasses

Some Types of Unit Cells

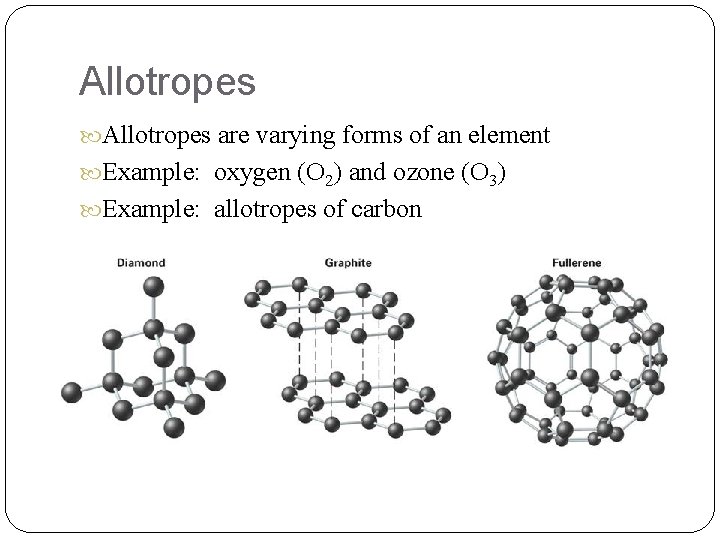
Allotropes are varying forms of an element Example: oxygen (O 2) and ozone (O 3) Example: allotropes of carbon

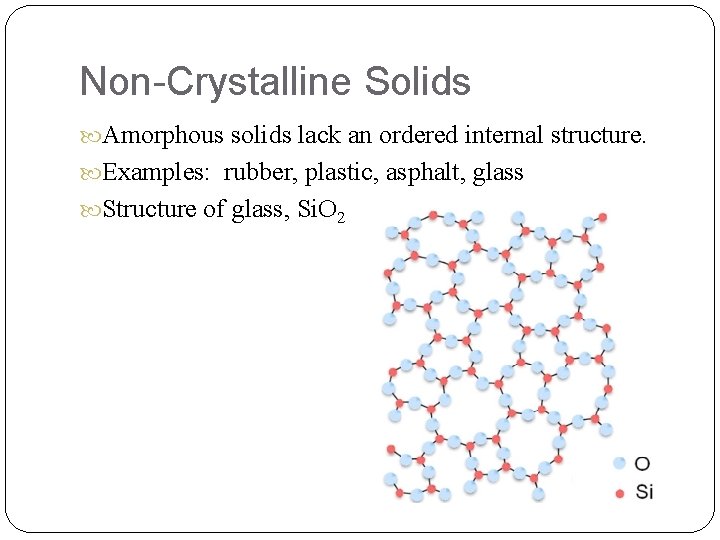
Non-Crystalline Solids Amorphous solids lack an ordered internal structure. Examples: rubber, plastic, asphalt, glass Structure of glass, Si. O 2

13. 4 Changes of State Vaporization/Condensation Liquid ↔ Gas Evaporation Boiling Vapor vs. Gas Melting/Freezing Liquid ↔ Solidification Sublimation/Deposition Solid ↔ Vapor I 2 (s) → I 2 (g)

Sublimation Solid Vapor Sublimation occurs in solids with vapor pressures that exceed atmospheric pressure at or near room temperature. Deposition o Vapor Solid

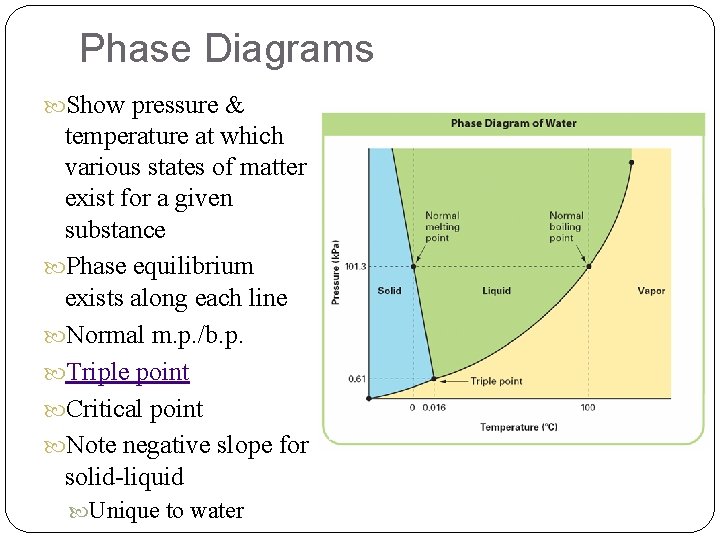
Phase Diagrams Show pressure & temperature at which various states of matter exist for a given substance Phase equilibrium exists along each line Normal m. p. /b. p. Triple point Critical point Note negative slope for solid-liquid Unique to water

Phase Diagrams A phase diagram is a graph that gives the conditions of temperature and pressure at which a substance exists as solid, liquid, and gas (vapor). Lines represent pressures and temperatures at which two phases are in equilibrium
 Greatest common factor of 18 and 27
Greatest common factor of 18 and 27 Common anode and common cathode
Common anode and common cathode Lcm questions
Lcm questions What are the factors for 54
What are the factors for 54 Common factors of 12 and 42
Common factors of 12 and 42 Multiples of 9 and 21
Multiples of 9 and 21 General property of matter
General property of matter States of matter foldable
States of matter foldable Four states of matter
Four states of matter Four states of matter
Four states of matter 5 states of matter
5 states of matter Thermal energy in states of matter
Thermal energy in states of matter Changing state
Changing state Phet states of matter basics
Phet states of matter basics 5 states of matter
5 states of matter Solid and liquid venn diagram
Solid and liquid venn diagram The kinetic theory of matter states that
The kinetic theory of matter states that Slave states free states
Slave states free states Southern vs northern states
Southern vs northern states Section 1 composition of matter
Section 1 composition of matter Chapter 12 states of matter study guide
Chapter 12 states of matter study guide Chapter 10 review states of matter section 4
Chapter 10 review states of matter section 4 States of matter: basics
States of matter: basics States of matter foldable
States of matter foldable Thermal energy vs heat
Thermal energy vs heat Uses of heat
Uses of heat The fundamental difference between states of matter is the
The fundamental difference between states of matter is the States of matter graph
States of matter graph Plasma to gas
Plasma to gas Grey matter reliaquest
Grey matter reliaquest