KineticMolecular Theory Kinetic Molecular Theory KMT is a



















- Slides: 25

Kinetic-Molecular Theory §Kinetic Molecular Theory (KMT) is a model used to explain the behavior of gases.

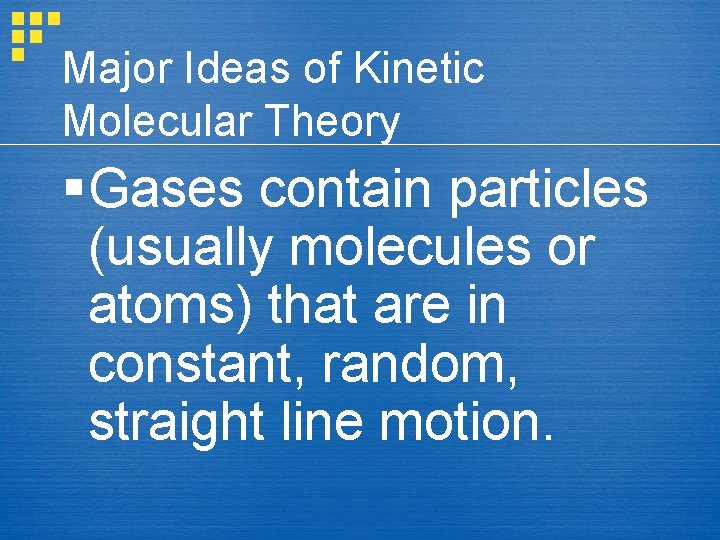
Major Ideas of Kinetic Molecular Theory § Gases contain particles (usually molecules or atoms) that are in constant, random, straight line motion.

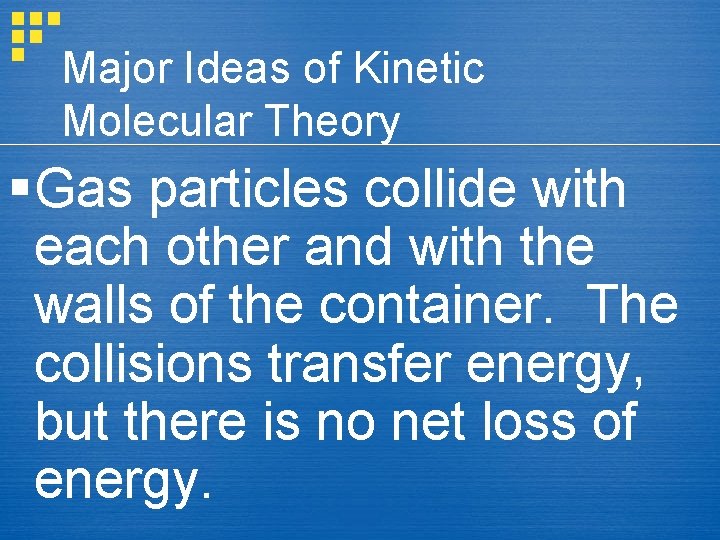
Major Ideas of Kinetic Molecular Theory § Gas particles collide with each other and with the walls of the container. The collisions transfer energy, but there is no net loss of energy.

Major Ideas of Kinetic Molecular Theory § Gas particles are separated by relatively great distances. Because of this, the volume occupied by the particles themselves is negligible.

Major Ideas of Kinetic Molecular Theory § Gas particles do not attract each other.

The Nature of Gases § KMT Theory explains why gases exert pressure § Not only do the gas particles collide with each other, but they also collide with the walls.

The Nature of Gases § The collisions with the wall exert a force over the surface area of the wall. § That is they exert pressure on the wall… § Recall that pressure is a force per unit area.

Units of Pressure § We will use two different units for pressure. Atmospheres (atm) kilo. Pascals § Animation

Temperature § Recall that temperature is the average kinetic energy of the particles. § So, the higher the temp, the faster the particles are moving.

Behavior of Gases § There are four variables… # of gas particles present Pressure Temperature Volume § … that explain the behavior of gases.

Chemistry Lab Boyles Law

Animations

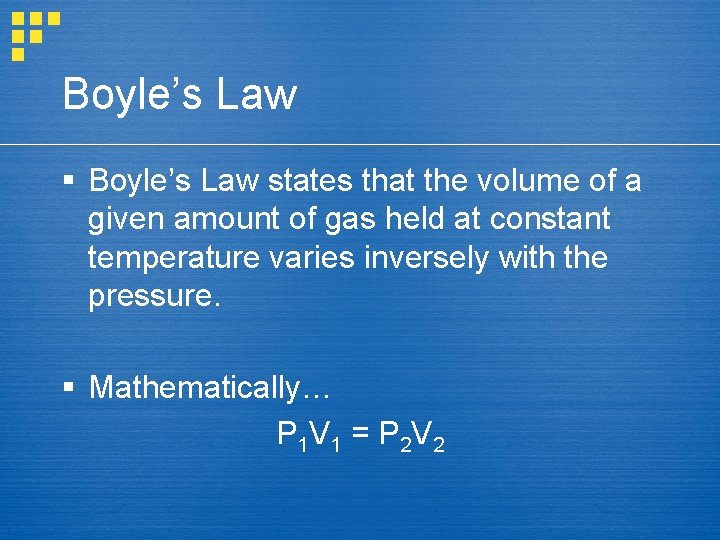
Boyle’s Law § Boyle’s Law states that the volume of a given amount of gas held at constant temperature varies inversely with the pressure. § Mathematically… P 1 V 1 = P 2 V 2

Chemistry Lab Pressure-Temperature Relationship in Gases

Gay-Lussac’s Law § States that the pressure of a given mass of gas varies directly with the kelvin temperature when the volume remains constant. § Mathematically, … P 1 / T 1 = P 2 / T 2

Practice Problem § A sealed rigid container has a volume of 2. 5 L, and a pressure of 2 atm at 400 K. What will be the new pressure if the container is cooled to 300 K?

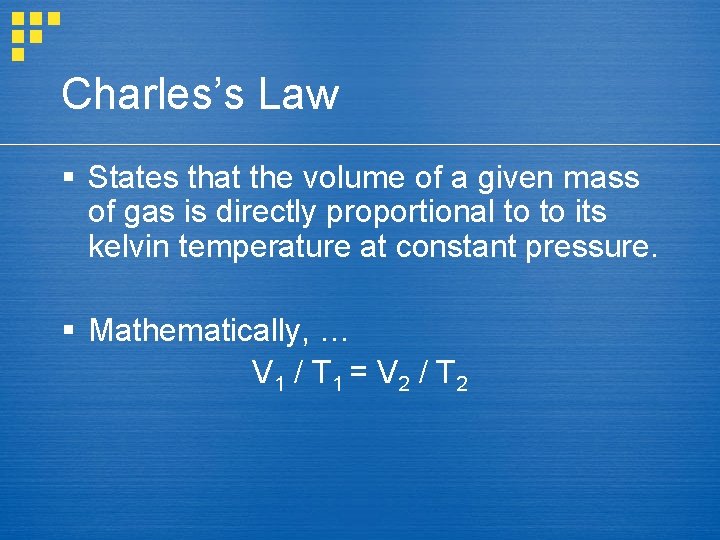
Charles’s Law § States that the volume of a given mass of gas is directly proportional to to its kelvin temperature at constant pressure. § Mathematically, … V 1 / T 1 = V 2 / T 2

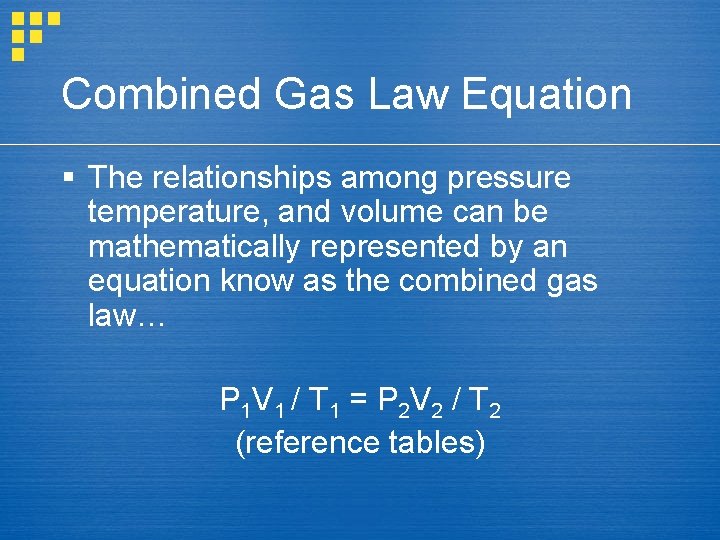
Combined Gas Law Equation § The relationships among pressure temperature, and volume can be mathematically represented by an equation know as the combined gas law… P 1 V 1 / T 1 = P 2 V 2 / T 2 (reference tables)

Practice Problem § What volume will a gas occupy if the pressure on 300 cm 3 of gas at 5. 0 atm is increased to 7. 0 atm? Assume the temperature remains constant.

Standard Temperature and Pressure § Standard pressure is defined as 1 atmosphere § One atmosphere is equal to… 101. 3 k. Pa 760 mm Hg 760 torr § Standard temperature is defined as 00 C (273 K)

Lets Practice More… § If 120 cm 3 of a gas is at STP, what volume will the gas occupy if the temperature is raised to 500 C and the pressure is increased to 950 torr?

Ideal vs. Real Gases § When gas laws are used to solve problems, the results don’t always agree exactly with lab results § The explanation is that the assumptions of KMT are not exactly correct.

Ideal vs. Real Gases § Gas particles really do attract one another! In most cases, IMF’s are so small they can be ignored But in extreme cases, the IMF’s become significant and important For example, water becomes snow or rain when the temps get low enough.

Ideal vs. Real Gases § Gas particles actually do occupy volume! As pressure increases, the volume occupied by the gas particles can’t be ignored. At high pressures, there are more frequent collisions.

Under What Conditions Is A Gas Nearly Ideal? § Low Pressure § High Temperature § Because those conditions allow the molecules to stay far away from one another.
 Kinetic molecular theory of solid
Kinetic molecular theory of solid Kinetic molecular theory
Kinetic molecular theory Kinetic molecular theory volume
Kinetic molecular theory volume Adhesive force
Adhesive force Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Postulates of kinetic molecular theory
Postulates of kinetic molecular theory Kinetic molecular theory def
Kinetic molecular theory def Theory vs hypothesis
Theory vs hypothesis Charles law in terms of kinetic molecular theory
Charles law in terms of kinetic molecular theory Kinetic molecular theory postulates
Kinetic molecular theory postulates Kinetic molecular theory
Kinetic molecular theory Postulates of kinetic theory of gases
Postulates of kinetic theory of gases Kinetic molecular theory formula
Kinetic molecular theory formula Kinetic molecular theory
Kinetic molecular theory Tenets of kinetic molecular theory
Tenets of kinetic molecular theory Principles of kmt
Principles of kmt Postulate 1
Postulate 1 Kmt
Kmt Kmt
Kmt Kmt gas laws
Kmt gas laws Kmt sct
Kmt sct Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Why molecular orbital theory is superior to vbt
Why molecular orbital theory is superior to vbt Molecular orbitals shapes
Molecular orbitals shapes