Gas Laws Kinetic Theory The kinetic theory of















































- Slides: 69

Gas Laws

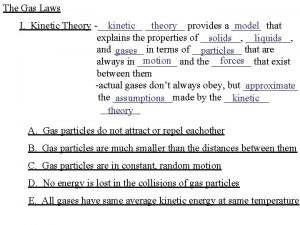
Kinetic Theory • The kinetic theory of gases is the study of the microscopic behavior of molecules • and the interactions which lead to macroscopic relationships like the ideal gas law.


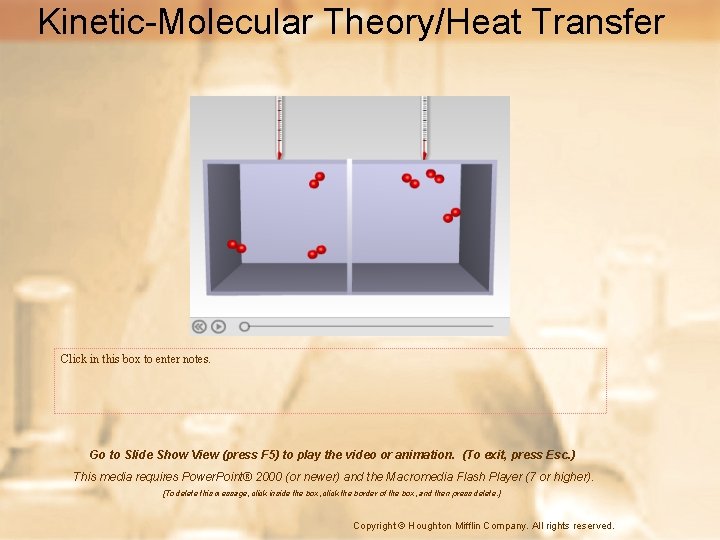
Kinetic-Molecular Theory/Heat Transfer Click in this box to enter notes. Go to Slide Show View (press F 5) to play the video or animation. (To exit, press Esc. ) This media requires Power. Point® 2000 (or newer) and the Macromedia Flash Player (7 or higher). [To delete this message, click inside the box, click the border of the box, and then press delete. ] Copyright © Houghton Mifflin Company. All rights reserved.

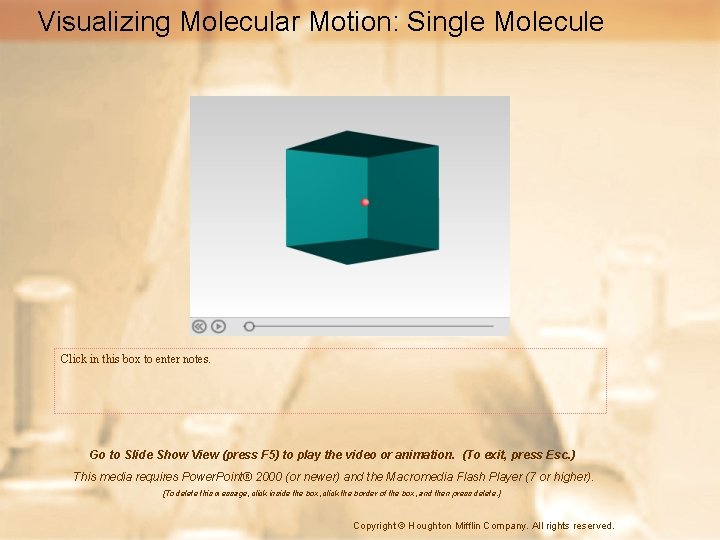
Visualizing Molecular Motion: Single Molecule Click in this box to enter notes. Go to Slide Show View (press F 5) to play the video or animation. (To exit, press Esc. ) This media requires Power. Point® 2000 (or newer) and the Macromedia Flash Player (7 or higher). [To delete this message, click inside the box, click the border of the box, and then press delete. ] Copyright © Houghton Mifflin Company. All rights reserved.

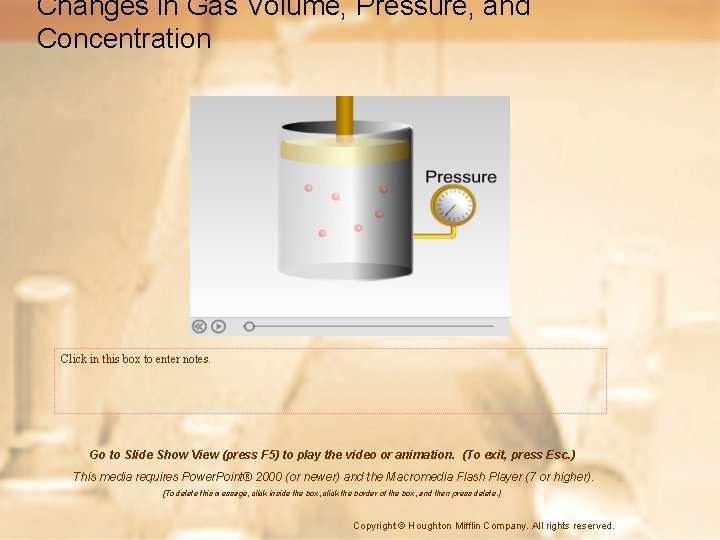
Gas Pressure • Pressure is defined as force per unit area. • Gas particles exert pressure when they collide with the walls of their container. • The SI unit of pressure is the pascal (Pa). • However, there are several units of pressure at STP – – – 0. 1013 Pascal (Pa) 101. 3 Kilopascal (KPa) 1 Atmosphere (atm) 760 mm. Hg 760 Torr 14. 7 lb/in 2

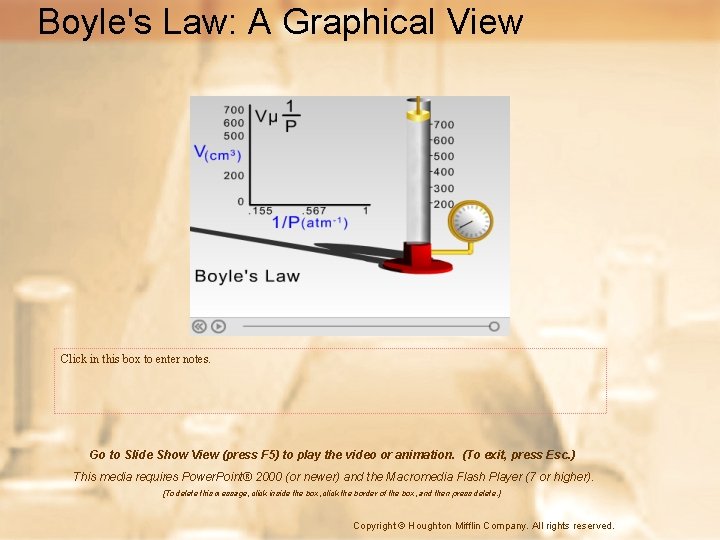
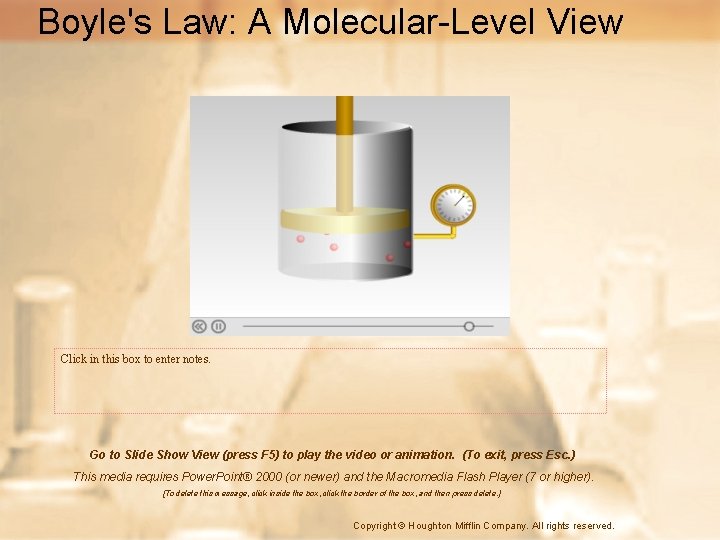
Boyle’s Law: Pressure and Volume • Boyle was an Irish chemist who studied the relationship between volume and pressure • Boyle’s law states that the pressure and volume of a gas at constant temperature are inversely proportional.

Boyle’s Law: Pressure and Volume • At a constant temperature, the pressure exerted by a gas depends on the frequency of collisions between gas particles and the container. • If the same number of particles is squeezed into a smaller space, the frequency of collisions increases, thereby increasing the pressure.

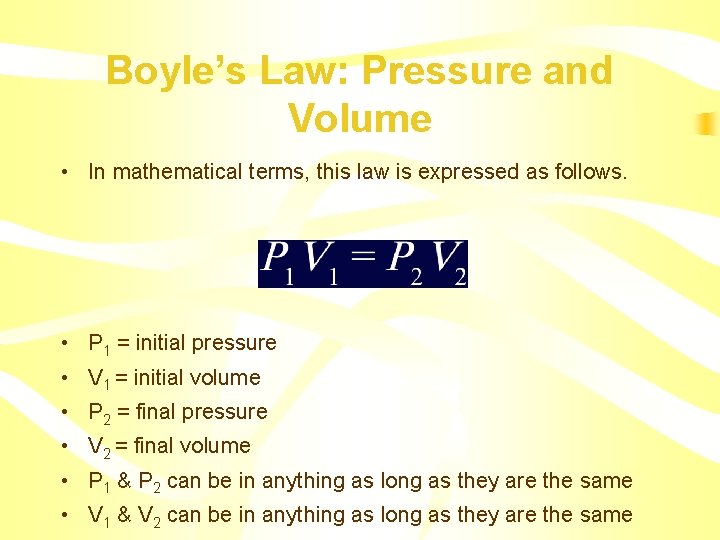
Boyle’s Law: Pressure and Volume • In mathematical terms, this law is expressed as follows. • P 1 = initial pressure • V 1 = initial volume • P 2 = final pressure • V 2 = final volume • P 1 & P 2 can be in anything as long as they are the same • V 1 & V 2 can be in anything as long as they are the same

Boyle's Law: A Graphical View Click in this box to enter notes. Go to Slide Show View (press F 5) to play the video or animation. (To exit, press Esc. ) This media requires Power. Point® 2000 (or newer) and the Macromedia Flash Player (7 or higher). [To delete this message, click inside the box, click the border of the box, and then press delete. ] Copyright © Houghton Mifflin Company. All rights reserved.

Boyle's Law: A Molecular-Level View Click in this box to enter notes. Go to Slide Show View (press F 5) to play the video or animation. (To exit, press Esc. ) This media requires Power. Point® 2000 (or newer) and the Macromedia Flash Player (7 or higher). [To delete this message, click inside the box, click the border of the box, and then press delete. ] Copyright © Houghton Mifflin Company. All rights reserved.

Changes in Gas Volume, Pressure, and Concentration Click in this box to enter notes. Go to Slide Show View (press F 5) to play the video or animation. (To exit, press Esc. ) This media requires Power. Point® 2000 (or newer) and the Macromedia Flash Player (7 or higher). [To delete this message, click inside the box, click the border of the box, and then press delete. ] Copyright © Houghton Mifflin Company. All rights reserved.

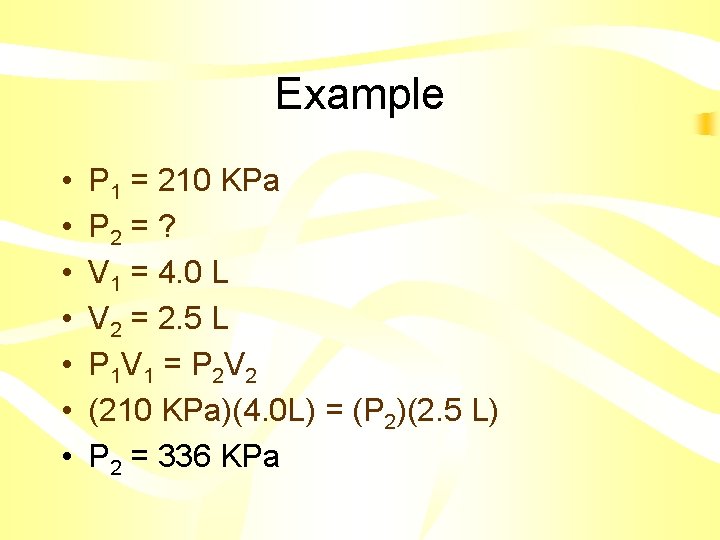
Example • A sample of Helium gas is compressed from 4. 0 L to 2. 5 L at a constant temperature. If the pressure of the gas in the 4. 0 L volume is 210 KPa, what will the pressure be at 2. 5 L?

Example • • P 1 = 210 KPa P 2 = ? V 1 = 4. 0 L V 2 = 2. 5 L P 1 V 1 = P 2 V 2 (210 KPa)(4. 0 L) = (P 2)(2. 5 L) P 2 = 336 KPa

Charles’ Law: Volume & Temperature • Charles was a French physicist who looked at the relationship between temperature and volume • He noted that as temperature went up, so did volume when pressure was held constant

Charles’ Law: Volume & Temperature • This observation is Charles’s law, which can be stated mathematically as follows.

Charles’ Law: Volume & Temperature • V 1 = V 2 T 1 T 2 • V 1 = initial volume • V 2 = final volume • T 1 = initial temperature • T 2 = final temperature • V 1 & V 2 can be in any unit as long as they are the same • T 1 & T 2 MUST be in Kelvin

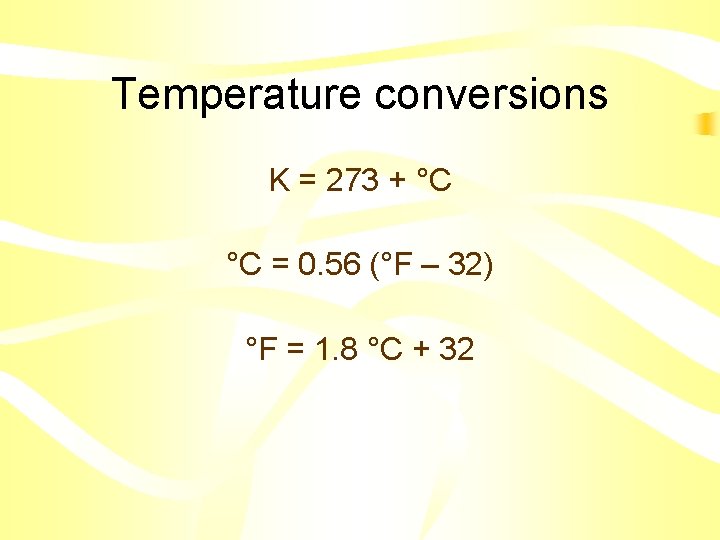
Temperature conversions K = 273 + °C °C = 0. 56 (°F – 32) °F = 1. 8 °C + 32

Charles's Law: A Graphical View Click in this box to enter notes. Go to Slide Show View (press F 5) to play the video or animation. (To exit, press Esc. ) This media requires Power. Point® 2000 (or newer) and the Macromedia Flash Player (7 or higher). [To delete this message, click inside the box, click the border of the box, and then press delete. ] Copyright © Houghton Mifflin Company. All rights reserved.

Charles's Law: A Molecular-Level View Click in this box to enter notes. Go to Slide Show View (press F 5) to play the video or animation. (To exit, press Esc. ) This media requires Power. Point® 2000 (or newer) and the Macromedia Flash Player (7 or higher). [To delete this message, click inside the box, click the border of the box, and then press delete. ] Copyright © Houghton Mifflin Company. All rights reserved.

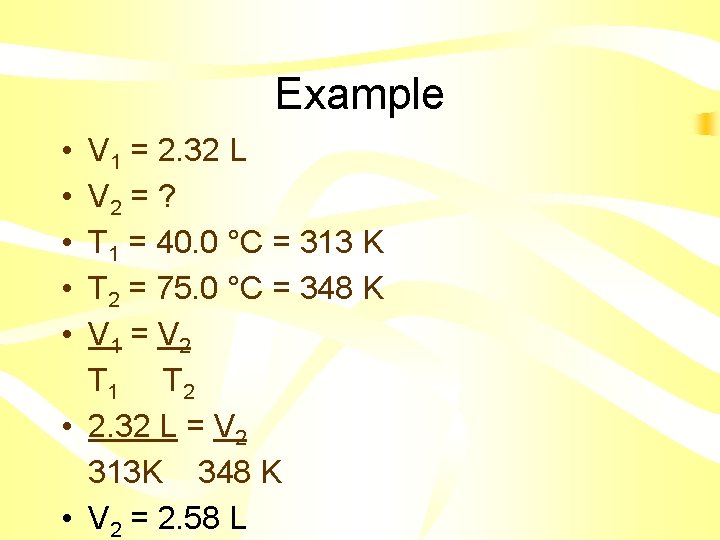
Example • A sample of gas at 40. 0 °C occupies a volume of 2. 32 L. If the temperature is raised to 75. 0 °C what will the new volume be?

Example • • • V 1 = 2. 32 L V 2 = ? T 1 = 40. 0 °C = 313 K T 2 = 75. 0 °C = 348 K V 1 = V 2 T 1 T 2 • 2. 32 L = V 2 313 K 348 K • V 2 = 2. 58 L

Gay Lussac’s Law: Pressure & Temperature • Gay Lussac studied the relationship between pressure and temperature • He noticed that at a constant volume a direct relationship existed between the Kelvin temperature and pressure • Giving the equation: • P 1 = P 2 T 1 T 2

Gay Lussac’s Law: Pressure & Temperature • P 1 = P 2 T 1 T 2 • P 1 = initial pressure • P 2 = final pressure • T 1 = initial temperature • T 2 = final temperature • P 1 & P 2 can be in any unit as long as they are the same • T 1 & T 2 MUST be in Kelvin

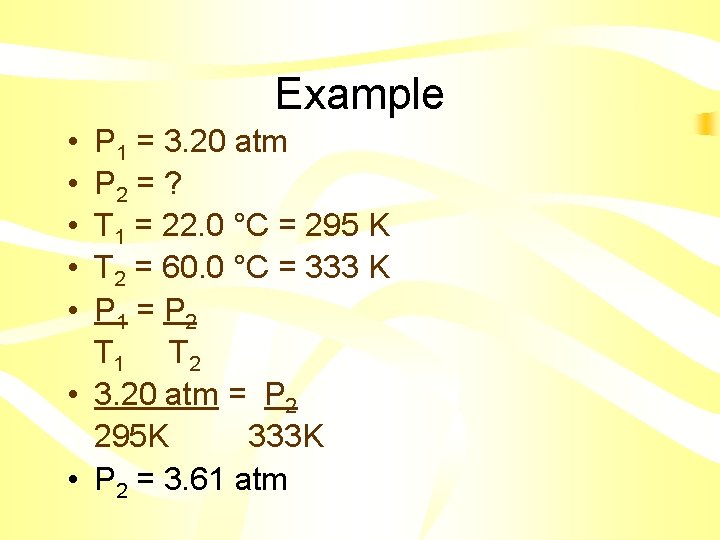
Example • The pressure of a gas in a tank is 3. 20 atm at 22. 0 °C. If the temperature rises to 60. 0 °C, what will the new pressure in the tank be?

Example • • • P 1 = 3. 20 atm P 2 = ? T 1 = 22. 0 °C = 295 K T 2 = 60. 0 °C = 333 K P 1 = P 2 T 1 T 2 • 3. 20 atm = P 2 295 K 333 K • P 2 = 3. 61 atm

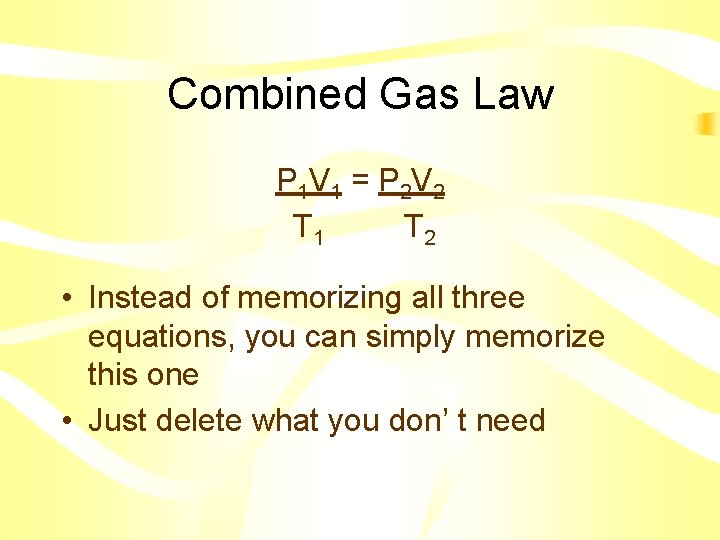
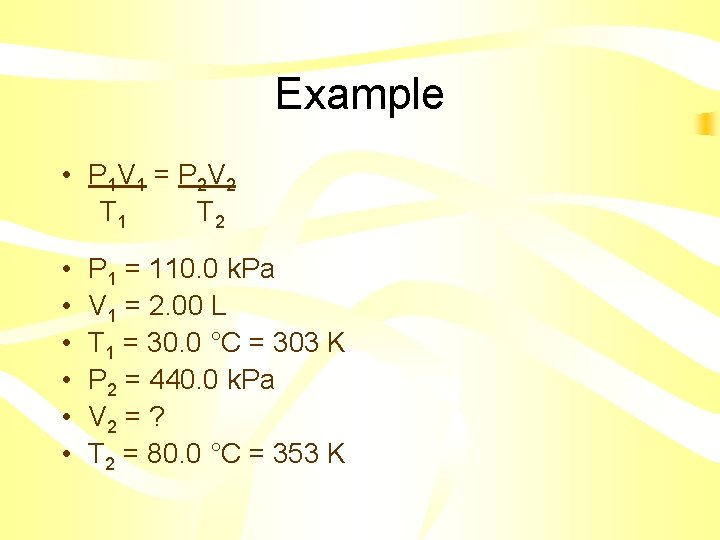
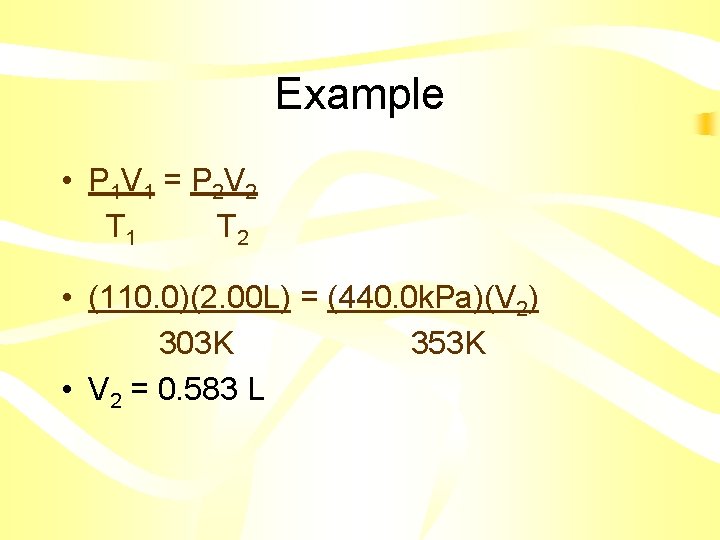
Combined Gas Law P 1 V 1 = P 2 V 2 T 1 T 2 • Instead of memorizing all three equations, you can simply memorize this one • Just delete what you don’ t need

Example • A gas at 110. 0 k. Pa and 30. 0°C fills a flexible container to a volume of 2. 00 L. If the temperature was raised to 80. 0°C and the pressure was increased to 440. 0 k. Pa, what is the new volume?

Example • P 1 V 1 = P 2 V 2 T 1 T 2 • • • P 1 = 110. 0 k. Pa V 1 = 2. 00 L T 1 = 30. 0 °C = 303 K P 2 = 440. 0 k. Pa V 2 = ? T 2 = 80. 0 °C = 353 K

Example • P 1 V 1 = P 2 V 2 T 1 T 2 • (110. 0)(2. 00 L) = (440. 0 k. Pa)(V 2) 303 K 353 K • V 2 = 0. 583 L

Ideal Gas Law & Gas Stoichiometry

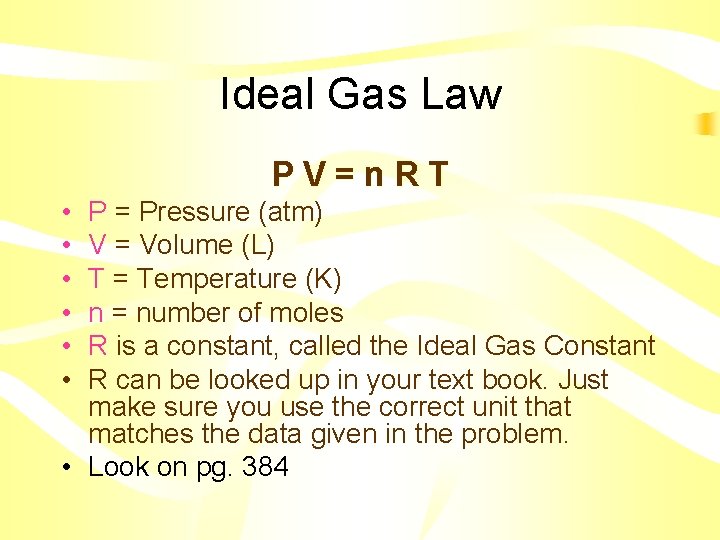
Ideal Gas Law PV=n. RT • • • P = Pressure (atm) V = Volume (L) T = Temperature (K) n = number of moles R is a constant, called the Ideal Gas Constant R can be looked up in your text book. Just make sure you use the correct unit that matches the data given in the problem. • Look on pg. 384

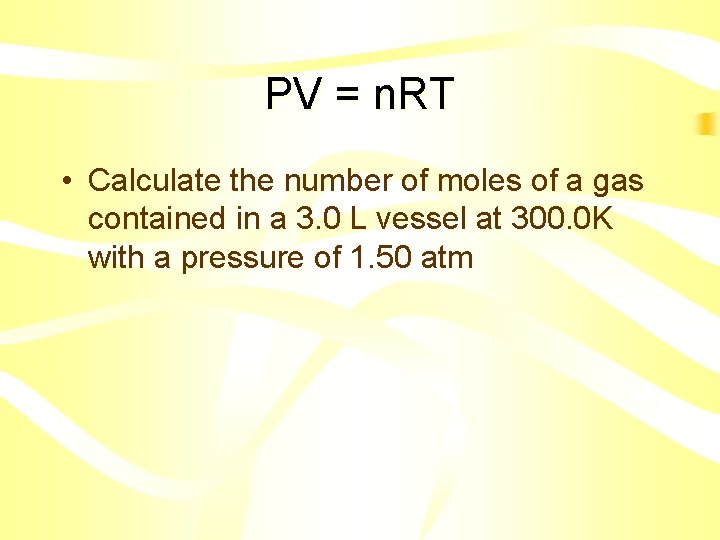
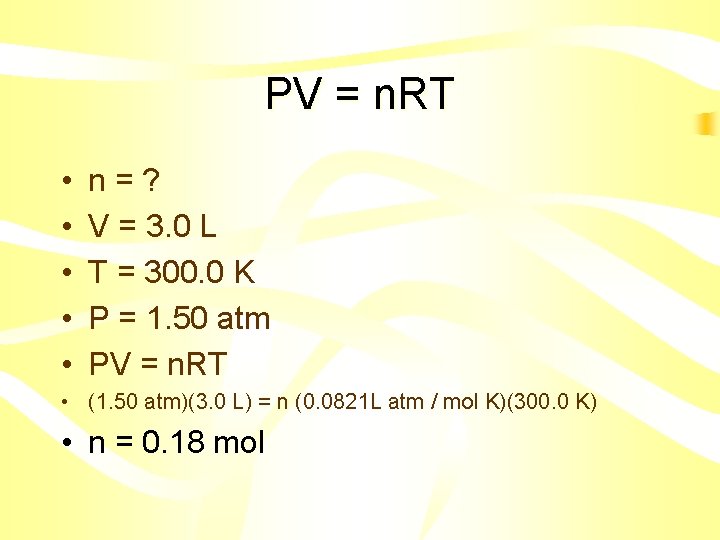
PV = n. RT • Calculate the number of moles of a gas contained in a 3. 0 L vessel at 300. 0 K with a pressure of 1. 50 atm

PV = n. RT • • • n=? V = 3. 0 L T = 300. 0 K P = 1. 50 atm PV = n. RT • (1. 50 atm)(3. 0 L) = n (0. 0821 L atm / mol K)(300. 0 K) • n = 0. 18 mol

The Ideal Gas Law, PV = n. RT Click in this box to enter notes. Go to Slide Show View (press F 5) to play the video or animation. (To exit, press Esc. ) This media requires Power. Point® 2000 (or newer) and the Macromedia Flash Player (7 or higher). [To delete this message, click inside the box, click the border of the box, and then press delete. ] Copyright © Houghton Mifflin Company. All rights reserved.

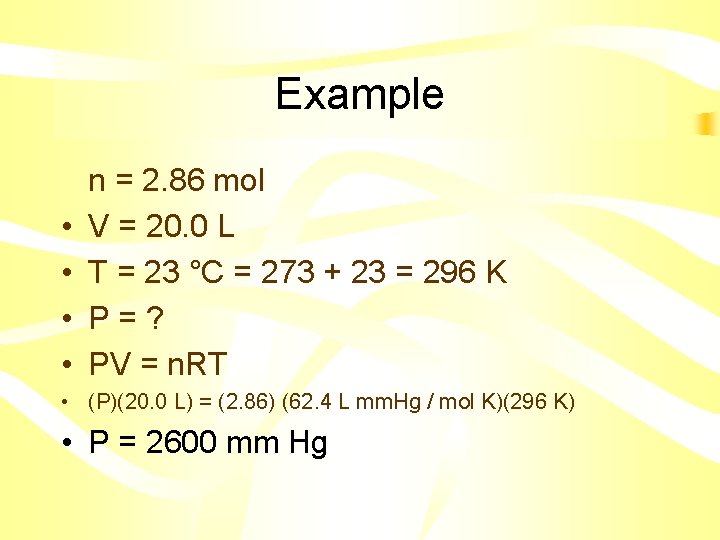
Example Dinitrogen monoxide (N 2 O), laughing gas, is used by dentists as an anesthetic. If 2. 86 mol of gas occupies a 20. 0 L tank at 23°C, what is the pressure (mm. Hg) in the tank in the dentist office?

Example • • n = 2. 86 mol V = 20. 0 L T = 23 °C = 273 + 23 = 296 K P=? PV = n. RT • (P)(20. 0 L) = (2. 86) (62. 4 L mm. Hg / mol K)(296 K) • P = 2600 mm Hg

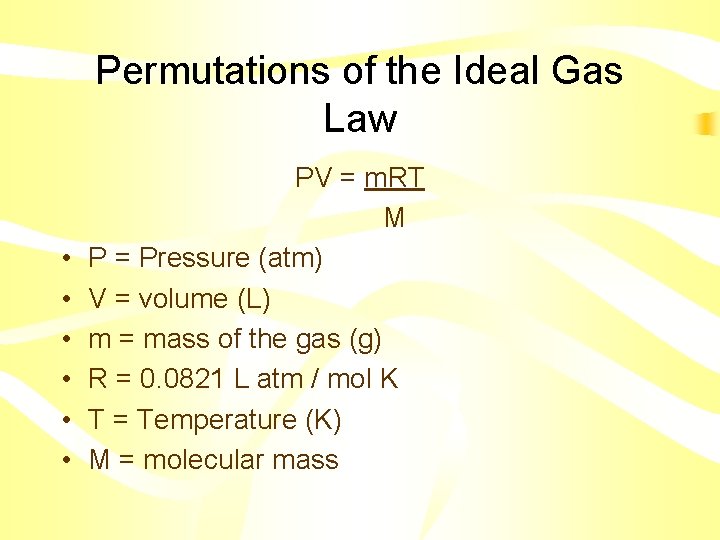
Permutations of the Ideal Gas Law • • • PV = m. RT M P = Pressure (atm) V = volume (L) m = mass of the gas (g) R = 0. 0821 L atm / mol K T = Temperature (K) M = molecular mass

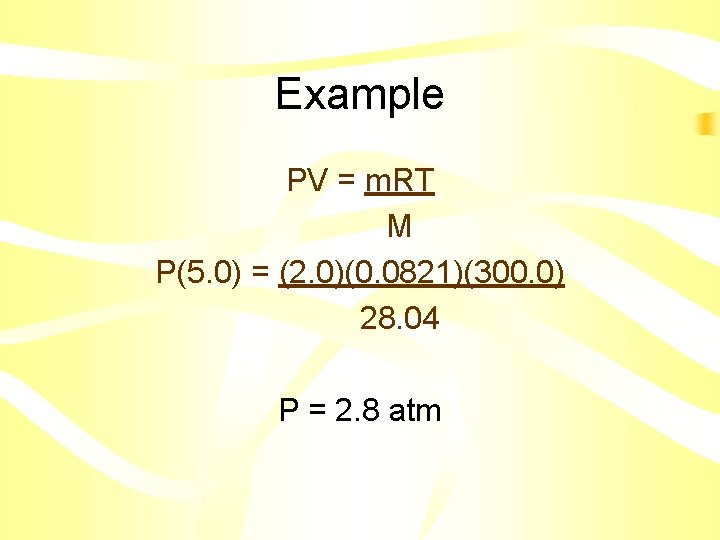
Example • What is the pressure 2. 0 g of nitrogen gas in a 5. 0 L container at 300. 0 K? • P=? • m = 2. 0 g • V = 5. 0 L • T = 300. 0 K • M = 28. 02 g/mol

Example PV = m. RT M P(5. 0) = (2. 0)(0. 0821)(300. 0) 28. 04 P = 2. 8 atm

Permutations of the Ideal Gas Law • • • P = DRT M P = pressure (atm) D = density (g/L) R = 0. 0821 L atm / mol K T = temperature (K) M = molecular mass

Example • What is the molar mass of a gas that has a density of 1. 40 g/L at STP? – NOTE – STP is standard temperature and pressure – At STP temperature is 273 K or 0 o. C and pressure is 1. 00 atm or any other equivalent unit.

Example • • • P = 1. 00 atm D = 1. 40 g/L R = 0. 0821 L atm / mol K T = 273 K M=? P = DRT M 1. 00 = (1. 40)(0. 0821)(273) M M = 31. 4 g/mol

Avogadro’s Principle • Avogadro’s Principle – equal volumes of gases at equal temperature and pressure contain the same number of particles • Molar volume – the volume of gas that 1 mole of a substance occupies at STP • At STP 1 mol of a gas = 22. 4 L • New conversion factor at STP ONLY! 1 mol 22. 4 L

Example • Calculate the volume 0. 881 mol of a gas will occupy at STP. • 0. 881 mol x 22. 4 L = 19. 7 L 1 mol (You could also have worked this out with the ideal gas law equation)

Example • Calculate the volume that 2. 000 kg of methane would occupy at STP. • 2. 000 kg x 1 x 10 3 g x 1 mol x 22. 4 L = 1 kg 16. 05 g 1 mol • 2791 L CH 4

Diffusion of gases Click in this box to enter notes. Go to Slide Show View (press F 5) to play the video or animation. (To exit, press Esc. ) This media requires Power. Point® 2000 (or newer) and the Macromedia Flash Player (7 or higher). [To delete this message, click inside the box, click the border of the box, and then press delete. ] Copyright © Houghton Mifflin Company. All rights reserved.

Effusion of a Gas Click in this box to enter notes. Go to Slide Show View (press F 5) to play the video or animation. (To exit, press Esc. ) This media requires Power. Point® 2000 (or newer) and the Macromedia Flash Player (7 or higher). [To delete this message, click inside the box, click the border of the box, and then press delete. ] Copyright © Houghton Mifflin Company. All rights reserved.

Kinetic Theory • The kinetic theory of gases is the study of the microscopic behavior of molecules • and the interactions which lead to macroscopic relationships like the ideal gas law.


Kinetic-Molecular Theory/Heat Transfer Click in this box to enter notes. Go to Slide Show View (press F 5) to play the video or animation. (To exit, press Esc. ) This media requires Power. Point® 2000 (or newer) and the Macromedia Flash Player (7 or higher). [To delete this message, click inside the box, click the border of the box, and then press delete. ] Copyright © Houghton Mifflin Company. All rights reserved.

Visualizing Molecular Motion: Many Molecules Click in this box to enter notes. Go to Slide Show View (press F 5) to play the video or animation. (To exit, press Esc. ) This media requires Power. Point® 2000 (or newer) and the Macromedia Flash Player (7 or higher). [To delete this message, click inside the box, click the border of the box, and then press delete. ] Copyright © Houghton Mifflin Company. All rights reserved.

Kinetic-Molecular Theory/Heat Transfer Click in this box to enter notes. Go to Slide Show View (press F 5) to play the video or animation. (To exit, press Esc. ) This media requires Power. Point® 2000 (or newer) and the Macromedia Flash Player (7 or higher). [To delete this message, click inside the box, click the border of the box, and then press delete. ] Copyright © Houghton Mifflin Company. All rights reserved.

Visualizing Molecular Motion: Single Molecule Click in this box to enter notes. Go to Slide Show View (press F 5) to play the video or animation. (To exit, press Esc. ) This media requires Power. Point® 2000 (or newer) and the Macromedia Flash Player (7 or higher). [To delete this message, click inside the box, click the border of the box, and then press delete. ] Copyright © Houghton Mifflin Company. All rights reserved.

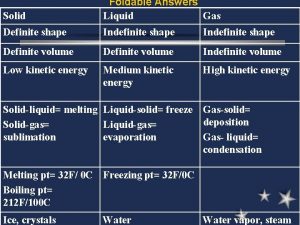
• What is a phase? • At its simplest, a phase can be just another term for solid, liquid or gas. If you have some ice floating in water, you have a solid phase present and a liquid phase. If there is air above the mixture, then that is another phase.

• Phase diagrams • A phase diagram lets you work out exactly what phases are present at any given temperature and pressure. In the cases we'll be looking at on this page, the phases will simply be the solid, liquid or vapor (gas) states of a pure substance.


The best way to remember which area corresponds to each of these states is to remember the conditions of temperature and pressure that are most likely to be associated with a solid, a liquid, and a gas. Low temperatures and high pressures favor the formation of a solid. Gases, on the other hand, are most likely to be found at high temperatures and low pressures. Liquids lie between these extremes.

• Along AB line: rate at which solid sublimes to form a gas=rate at which gas condenses to form a solid

• Along BC line: rate at which liquid boils to form a gas=rate at which gas condenses to form a liquid • The solid line between points B and D contains the combinations of temperature and pressure at

• Along BD line: rate at which solid melts to form a liquid=rate at which liquid freezes to form a solid

• Point B in this phase diagram represents the only combination of temperature and pressure at which a pure substance can exist simultaneously as a solid, a liquid, and a gas. It is therefore called the triple point of the substance, and it represents the only point in the phase diagram in which all three states are in equilibrium. Point C is the critical point of the substance, which is the highest temperature and pressure at which a gas and a liquid can coexist at equilibrium.

• In theory, we should be able to predict the pressure at which a gas condenses at a given temperature by consulting a plot of vapor pressure vs. temperature. In practice, every compound has a critical temperature (Tc). If the temperature of the gas is above the critical temperature, the gas can't be condensed, regardless of the pressure applied.

• Gases can't be liquified at temperatures above the critical temperature because at this point the properties of gases and liquids become the same, and there is no basis on which to distinguish between gases and liquids. The vapor pressure of a liquid at the critical temperature is called the critical pressure (Pc). The vapor pressure of a liquid never gets larger than this critical pressure.


Melting Point and Freezing Point • Pure, crystalline solids have a characteristic melting point, the temperature at which the solid melts to become a liquid. The transition between the solid and the liquid is so sharp for small samples of a pure substance that melting points can be measured to 0. 1 o. C. The melting point of solid oxygen, for example, is -218. 4 o. C.

• Liquids have a characteristic temperature at which they turn into solids, known as their freezing point. In theory, the melting point of a solid should be the same as the freezing point of the liquid. In practice, small differences between these quantities can be observed.

Boiling Point • When a liquid is heated, it eventually reaches a temperature at which the vapor pressure is large enough that bubbles form inside the body of the liquid. This temperature is called the boiling point. Once the liquid starts to boil, the temperature remains constant until all of the liquid has been converted to a gas.

• The normal boiling point of water is 100 o. C. But if you try to cook an egg in boiling water while camping in the Rocky Mountains at an elevation of 10, 000 feet, you will find that it takes longer for the egg to cook because water boils at only 90 o. C at this elevation.
 Postulates of kinetic theory of gases class 11
Postulates of kinetic theory of gases class 11 Facts about montesquieu
Facts about montesquieu Gas laws crash course
Gas laws crash course Direct or indirect relationship
Direct or indirect relationship Stp gas laws
Stp gas laws Combined gas law
Combined gas law All the gas laws
All the gas laws Different gas laws
Different gas laws How hot air balloons operate gas laws
How hot air balloons operate gas laws Gas laws conceptual questions
Gas laws conceptual questions Example of boyle's law
Example of boyle's law Combined gas law practice worksheet answers
Combined gas law practice worksheet answers A gas occupies 473 cm3 at 36°c. find its volume at 94°c
A gas occupies 473 cm3 at 36°c. find its volume at 94°c Different gas laws
Different gas laws Combined gas law worksheet
Combined gas law worksheet Chapter 13 gases
Chapter 13 gases Boyle's law states that
Boyle's law states that Charles law formula
Charles law formula Ap chemistry gas laws
Ap chemistry gas laws Gas laws graphic organizer
Gas laws graphic organizer Gas laws hot air balloon
Gas laws hot air balloon How to calculate boyle's law
How to calculate boyle's law Kmt gas laws
Kmt gas laws Gas law formulas
Gas law formulas Empirical gas laws
Empirical gas laws Charles law
Charles law Gas laws formulas
Gas laws formulas Is gas definite or indefinite
Is gas definite or indefinite Relation between pressure and kinetic energy of gas
Relation between pressure and kinetic energy of gas Kinetic energy of gas molecules
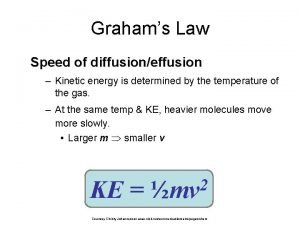
Kinetic energy of gas molecules Graham's law of diffusion
Graham's law of diffusion Derive ideal gas equation
Derive ideal gas equation An ideal gas is an imaginary gas
An ideal gas is an imaginary gas Differences between ideal gas and real gas
Differences between ideal gas and real gas Ideal gas vs perfect gas
Ideal gas vs perfect gas Conclusion of bhopal gas tragedy
Conclusion of bhopal gas tragedy Gas leaked in bhopal gas tragedy
Gas leaked in bhopal gas tragedy Volume molare
Volume molare Flue gas desulfurisation gas filter
Flue gas desulfurisation gas filter Poisonous gas leaked in bhopal gas tragedy
Poisonous gas leaked in bhopal gas tragedy Difference between ideal gas and real gas
Difference between ideal gas and real gas Contoh soal kinetika kimia orde 1
Contoh soal kinetika kimia orde 1 Gas exchange key events in gas exchange
Gas exchange key events in gas exchange Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Ng-html
Ng-html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Chụp phim tư thế worms-breton
Chụp phim tư thế worms-breton Chúa yêu trần thế
Chúa yêu trần thế Các môn thể thao bắt đầu bằng tiếng bóng
Các môn thể thao bắt đầu bằng tiếng bóng Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công của trọng lực
Công của trọng lực Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Phép trừ bù
Phép trừ bù độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế chỉ nói điều hay thôi
Cái miệng nó xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ V cc
V cc Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thẻ vin
Thẻ vin