Properties of Gases Kinetic Molecular Model Speed of
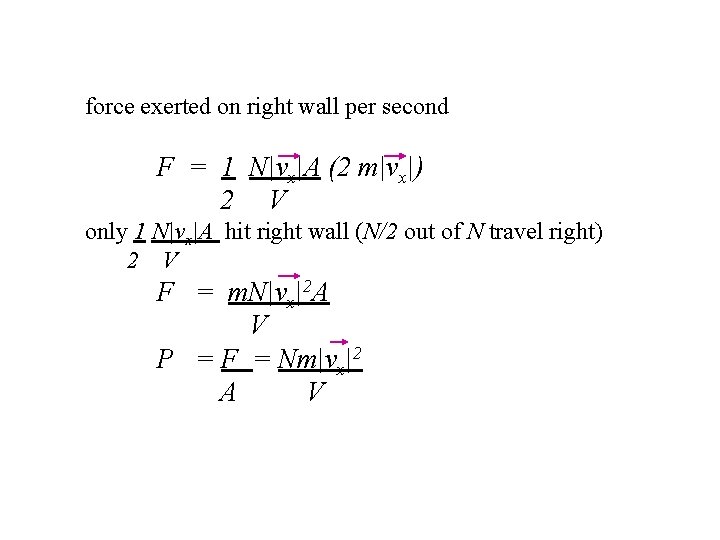
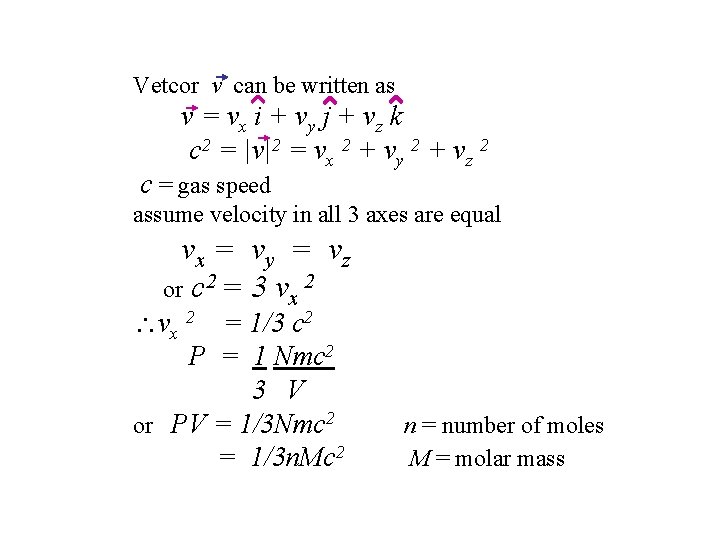
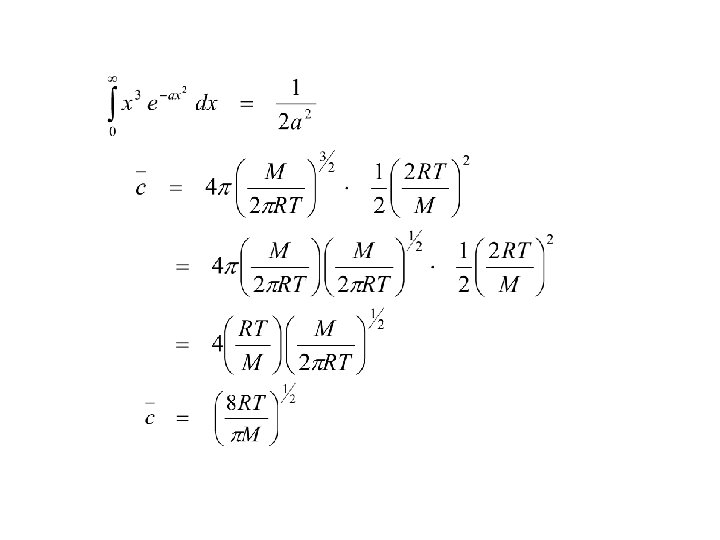
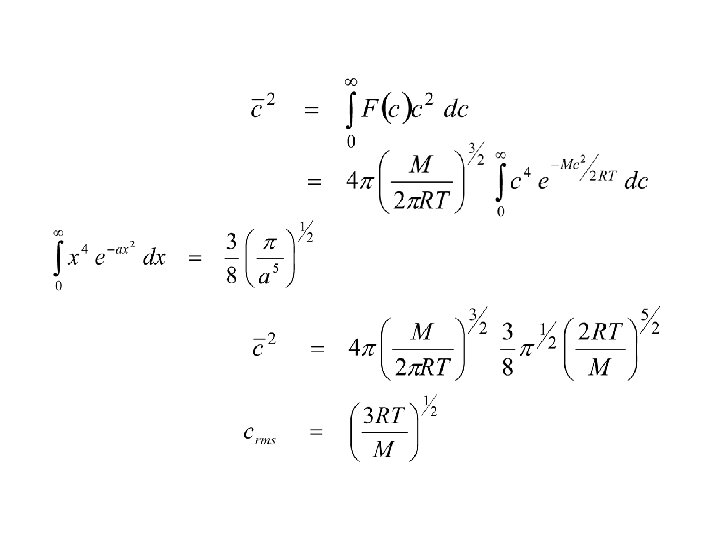


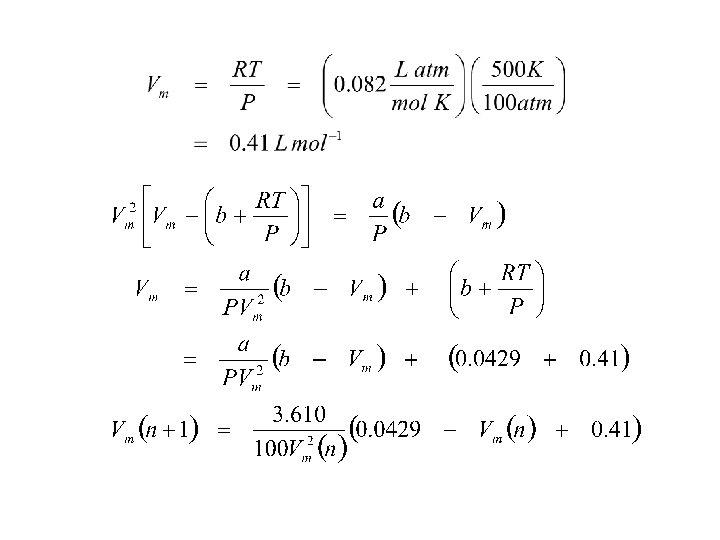
- Slides: 34

Properties of Gases • Kinetic Molecular Model • Speed of gas • Combining Kinetic Molecular Model and Ideal gas Law • Real gas • Virial equation • PV-plot for real gas • Van der Waals equation • Comparing ideal and real gas

Kinetic molecular model of Gases • Assumption 1) gas molecules in ceaseless random motion ( no phase change) 2) molecular size is negligible (no repulsion) 3) molecules do not interact (no attraction) All gases at same T and P have the same properties

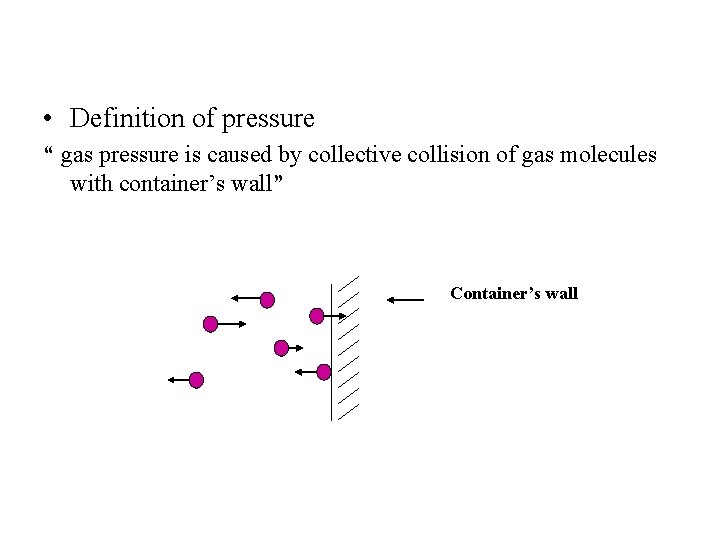
• Definition of pressure “ gas pressure is caused by collective collision of gas molecules with container’s wall” Container’s wall

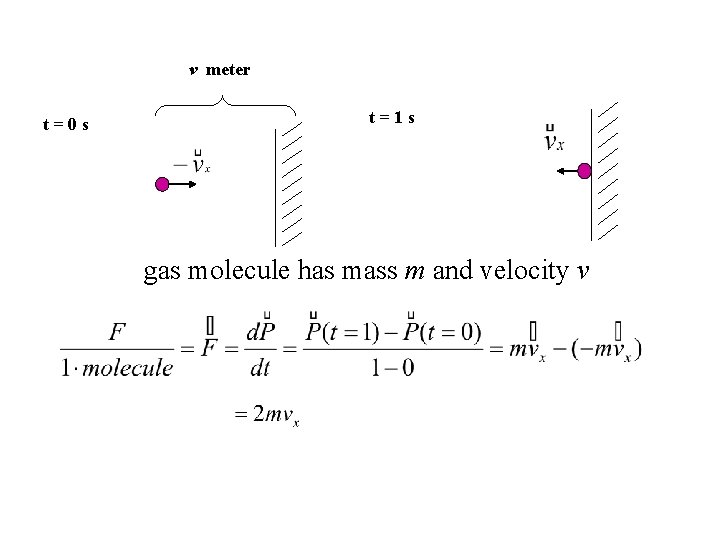
v meter t=0 s t=1 s gas molecule has mass m and velocity v

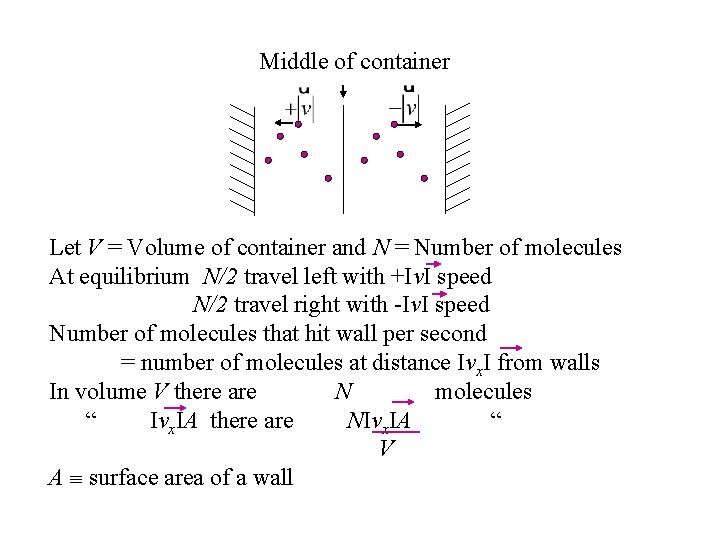
Middle of container Let V = Volume of container and N = Number of molecules At equilibrium N/2 travel left with +Iv. I speed N/2 travel right with -Iv. I speed Number of molecules that hit wall per second = number of molecules at distance Ivx. I from walls In volume V there are N molecules “ Ivx. IA there are NIvx. IA “ V A surface area of a wall
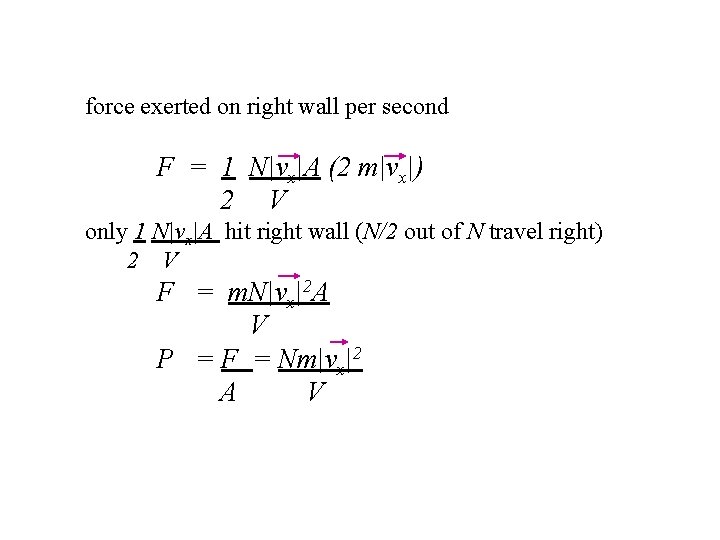
force exerted on right wall per second F = 1 N|vx|A (2 m|vx|) 2 V only 1 N|vx|A hit right wall (N/2 out of N travel right) 2 V F = m. N|vx|2 A V P = F = Nm|vx|2 A V
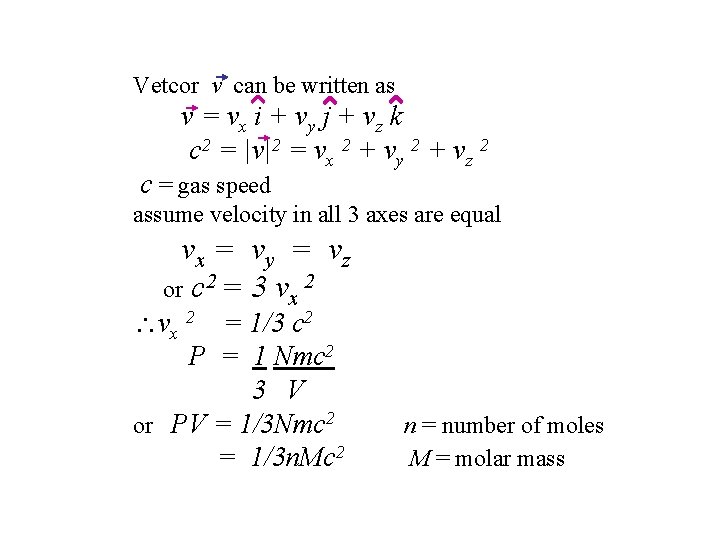
Vetcor v can be written as v = vx i + vy j + vz k c 2 = |v|2 = vx 2 + vy 2 + vz 2 c = gas speed assume velocity in all 3 axes are equal vx = v y = v z or c 2 = 3 vx 2 vx 2 = 1/3 c 2 P = 1 Nmc 2 3 V or PV = 1/3 Nmc 2 = 1/3 n. Mc 2 n = number of moles M = molar mass

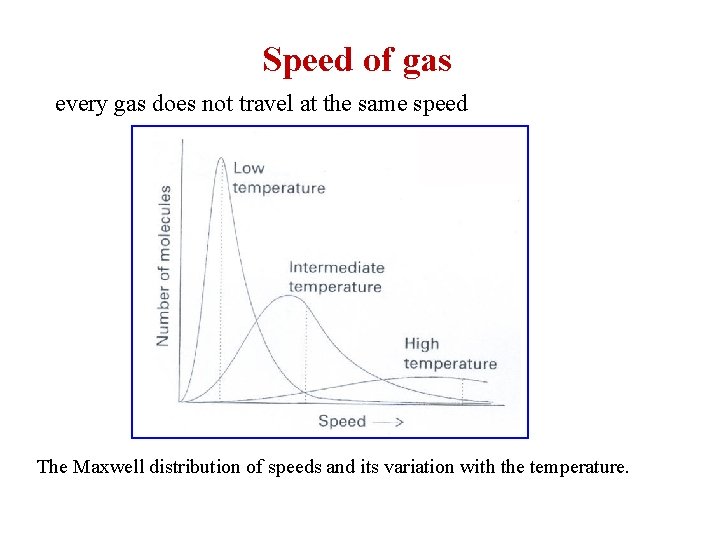
Speed of gas every gas does not travel at the same speed The Maxwell distribution of speeds and its variation with the temperature.

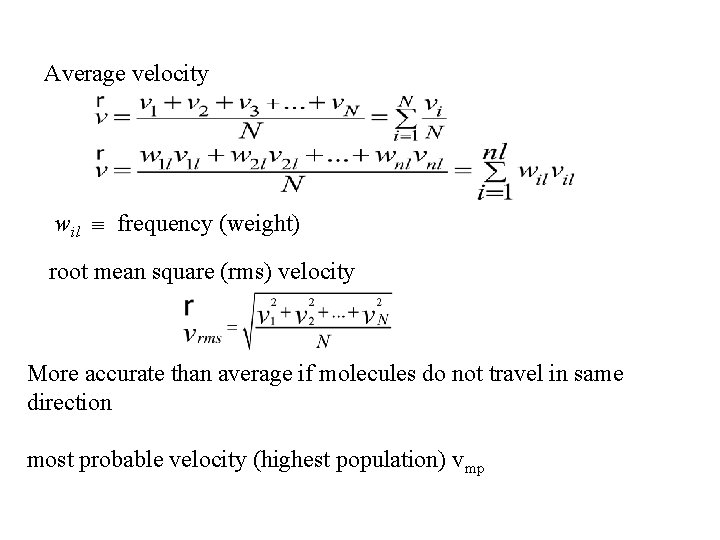
Average velocity wil frequency (weight) root mean square (rms) velocity More accurate than average if molecules do not travel in same direction most probable velocity (highest population) vmp

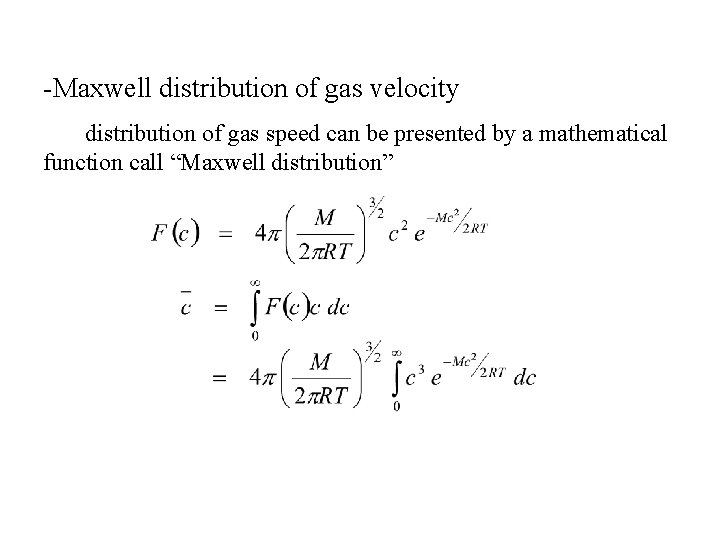
-Maxwell distribution of gas velocity distribution of gas speed can be presented by a mathematical function call “Maxwell distribution”
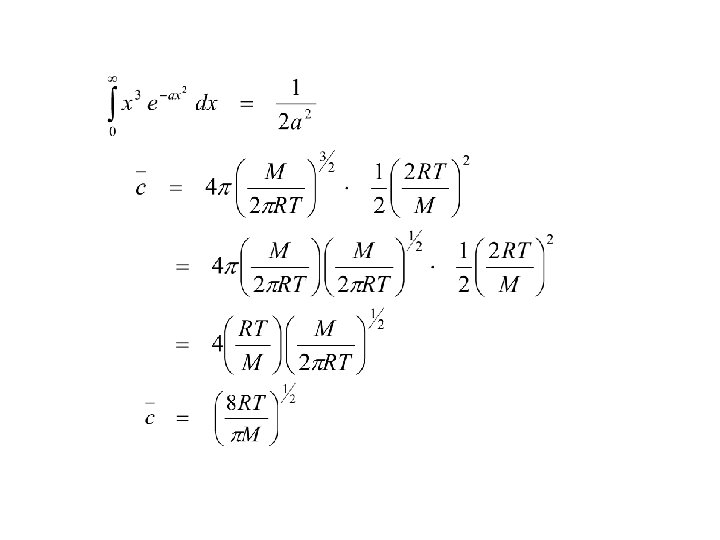
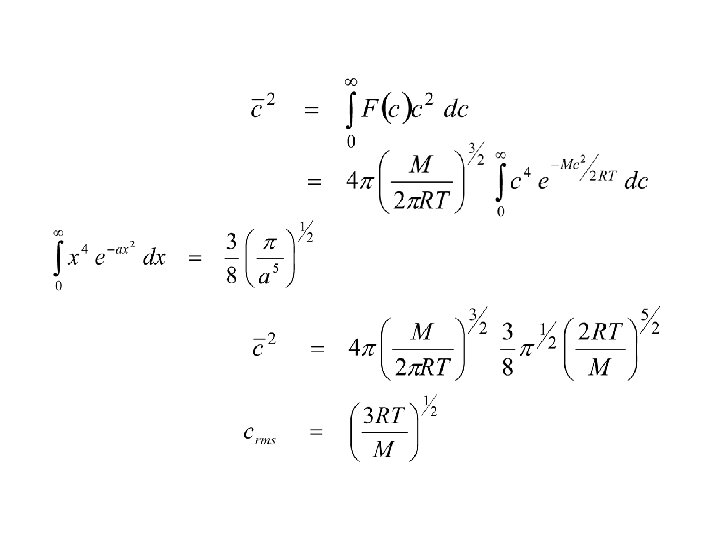

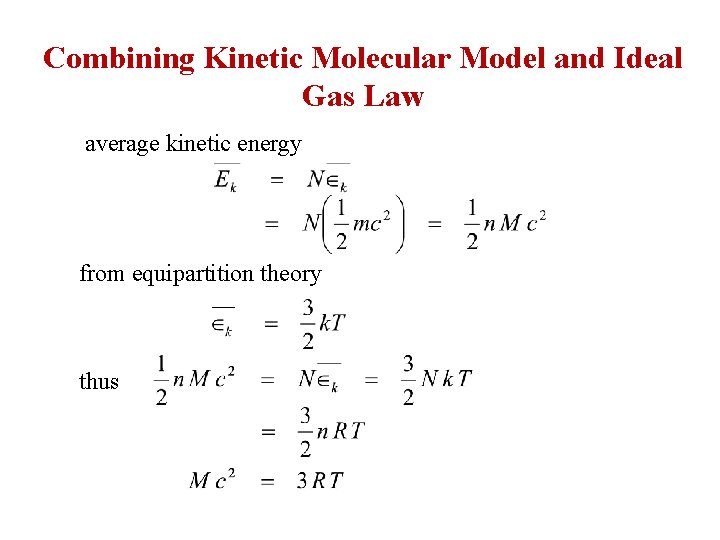
Combining Kinetic Molecular Model and Ideal Gas Law average kinetic energy from equipartition theory thus

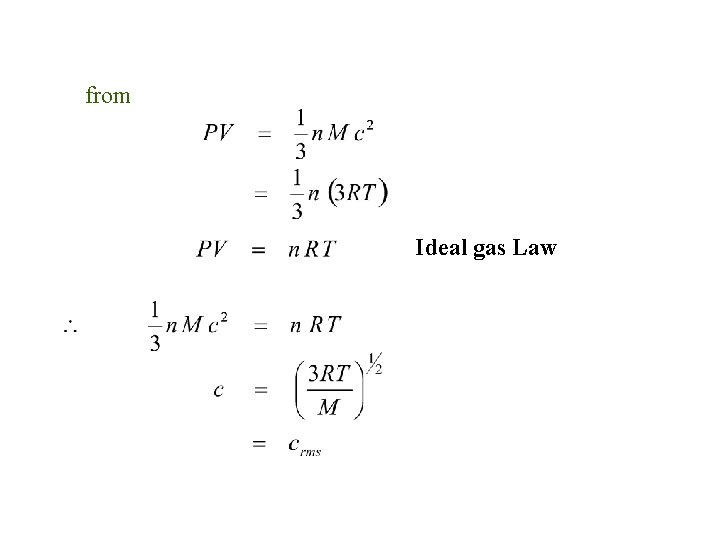
from Ideal gas Law

Real gas 1. molecules not always in motion (condense phase can be formed) 2. molecular size is non-negligible (there is molecular repulsion( 3. Molecules do interact (there is molecular attraction)

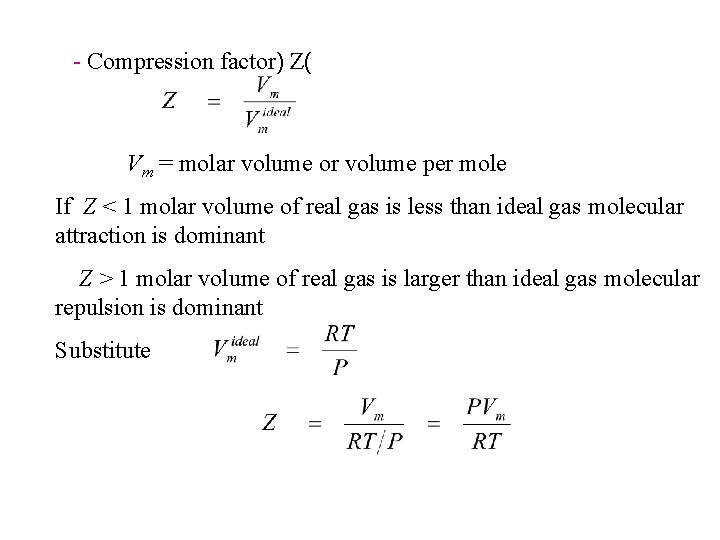
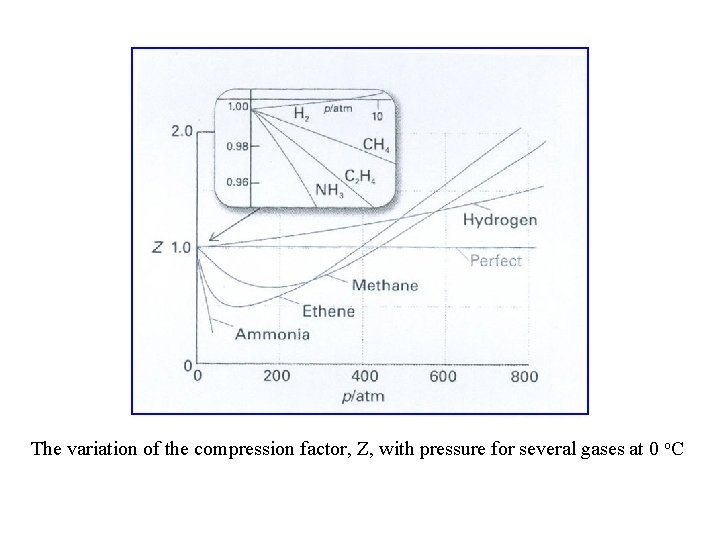
- Compression factor) Z( Vm = molar volume or volume per mole If Z < 1 molar volume of real gas is less than ideal gas molecular attraction is dominant Z > 1 molar volume of real gas is larger than ideal gas molecular repulsion is dominant Substitute

The variation of the compression factor, Z, with pressure for several gases at 0 o. C

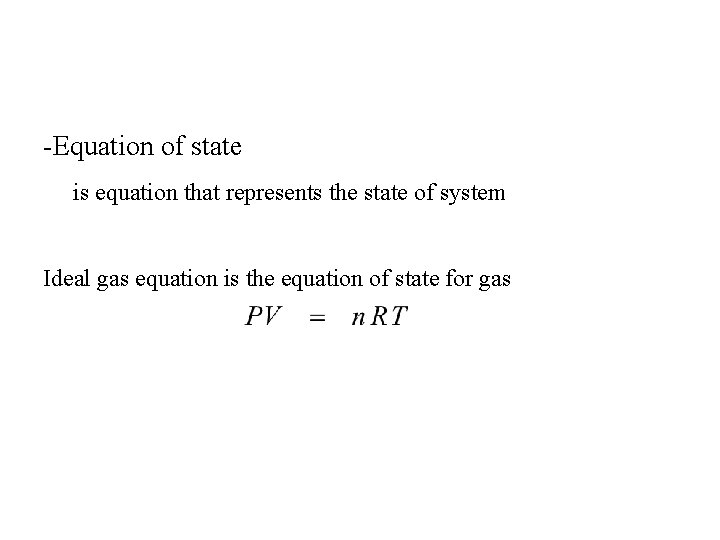
-Equation of state is equation that represents the state of system Ideal gas equation is the equation of state for gas

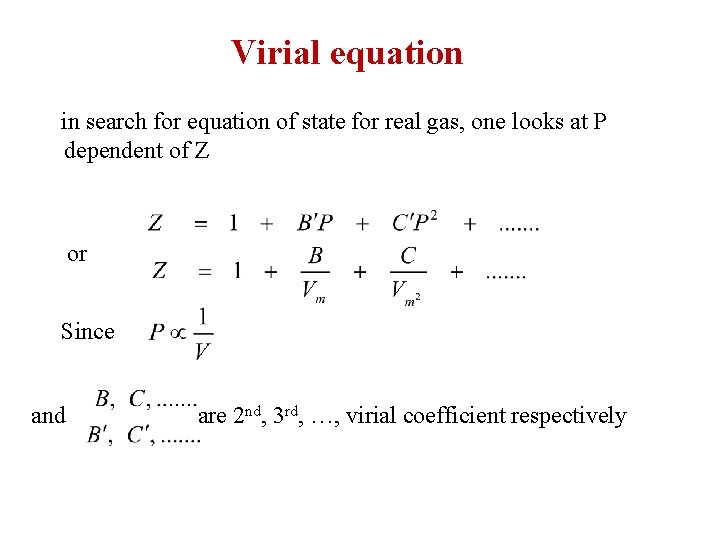
Virial equation in search for equation of state for real gas, one looks at P dependent of Z or Since and are 2 nd, 3 rd, …, virial coefficient respectively

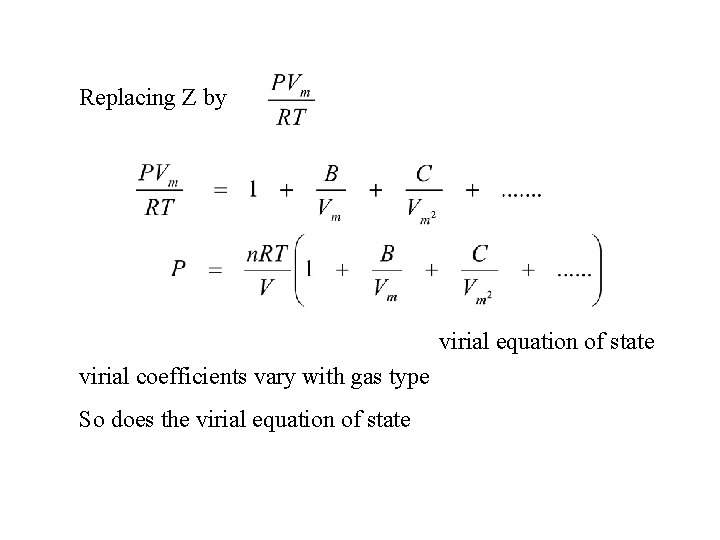
Replacing Z by virial equation of state virial coefficients vary with gas type So does the virial equation of state

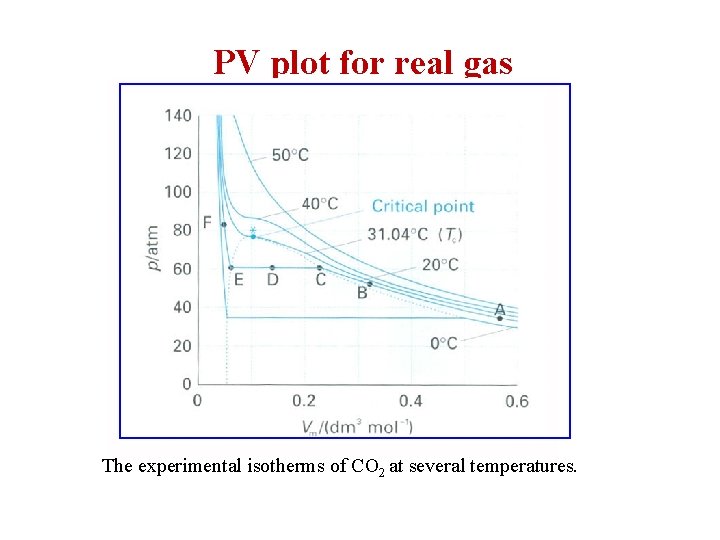
PV plot for real gas The experimental isotherms of CO 2 at several temperatures.

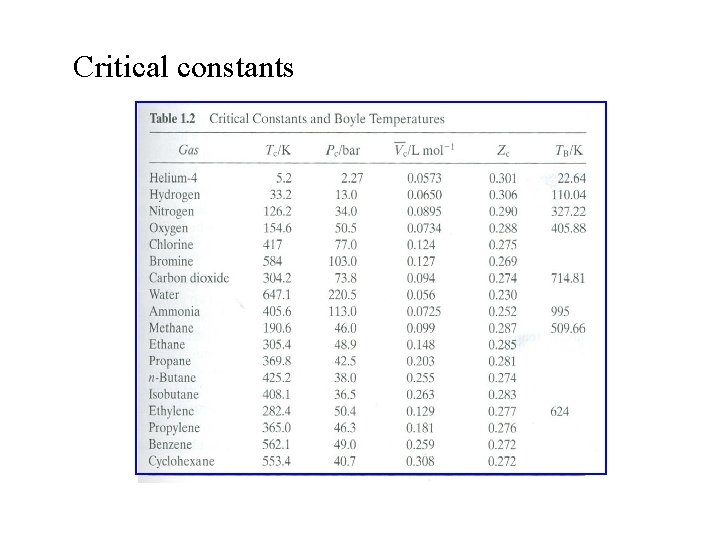
Critical constants

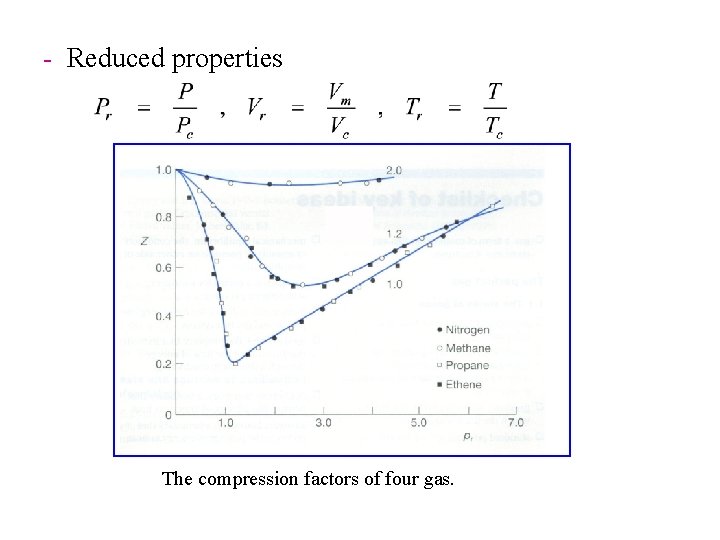
- Reduced properties The compression factors of four gas.

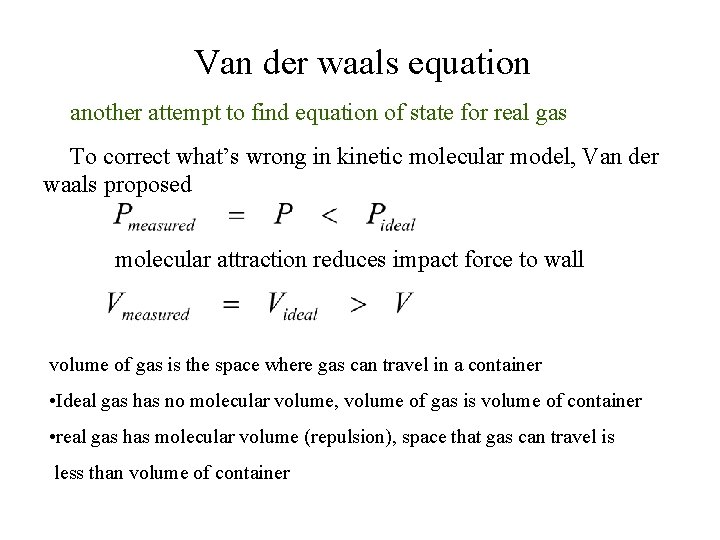
Van der waals equation another attempt to find equation of state for real gas To correct what’s wrong in kinetic molecular model, Van der waals proposed molecular attraction reduces impact force to wall volume of gas is the space where gas can travel in a container • Ideal gas has no molecular volume, volume of gas is volume of container • real gas has molecular volume (repulsion), space that gas can travel is less than volume of container

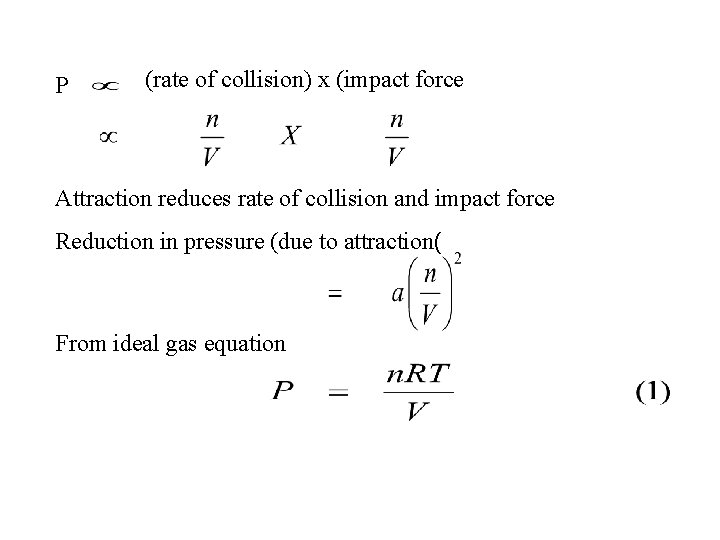
P (rate of collision) x (impact force Attraction reduces rate of collision and impact force Reduction in pressure (due to attraction( From ideal gas equation

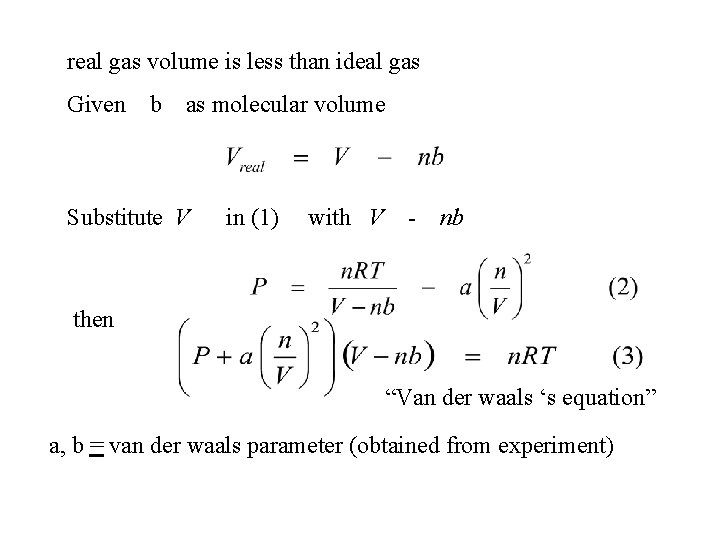
real gas volume is less than ideal gas Given b as molecular volume Substitute V in (1) with V - nb then “Van der waals ‘s equation” a, b = van der waals parameter (obtained from experiment)

Since Van der Waals equation comes from modification of kinetic molecular model (theory) while a, b comes from experiment Van der waals equation is “semi-empirical ” virial equation is “empirical ”

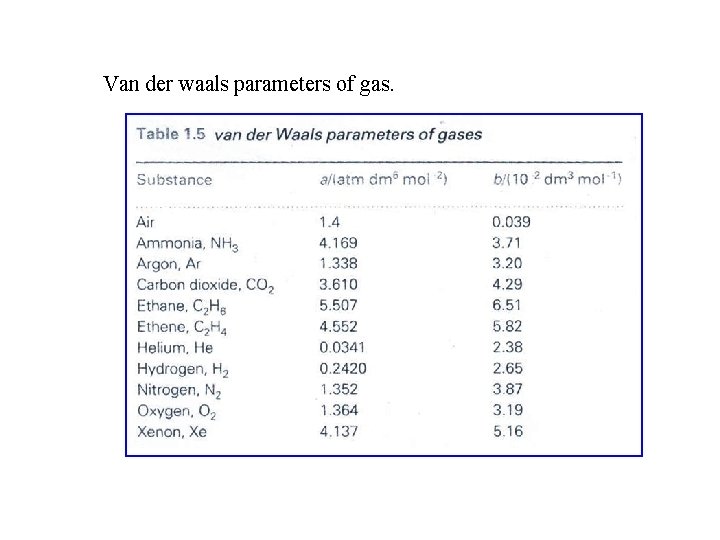
Van der waals parameters of gas.

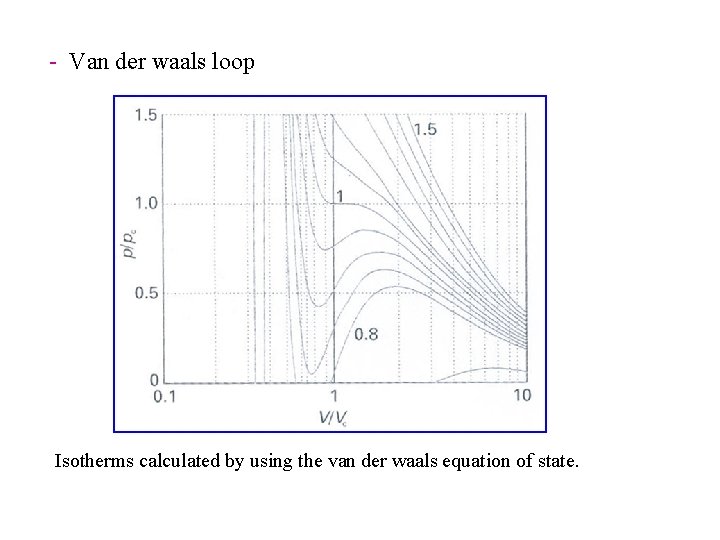
- Van der waals loop Isotherms calculated by using the van der waals equation of state.

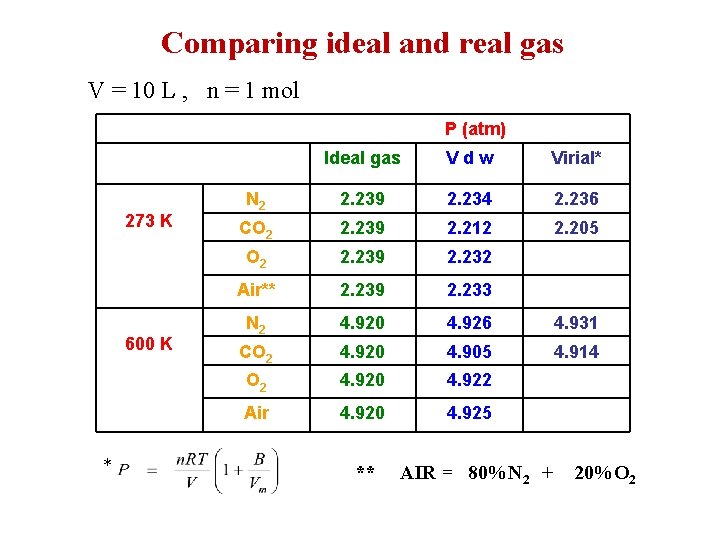
Comparing ideal and real gas V = 10 L , n = 1 mol P (atm) 273 K 600 K * Ideal gas Vdw Virial* N 2 2. 239 2. 234 2. 236 CO 2 2. 239 2. 212 2. 205 O 2 2. 239 2. 232 Air** 2. 239 2. 233 N 2 4. 920 4. 926 4. 931 CO 2 4. 920 4. 905 4. 914 O 2 4. 920 4. 922 Air 4. 920 4. 925 ** AIR = 80%N 2 + 20%O 2

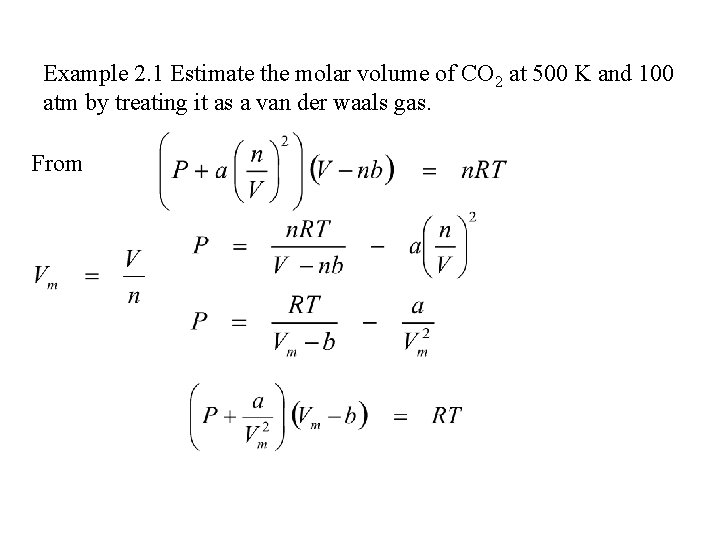
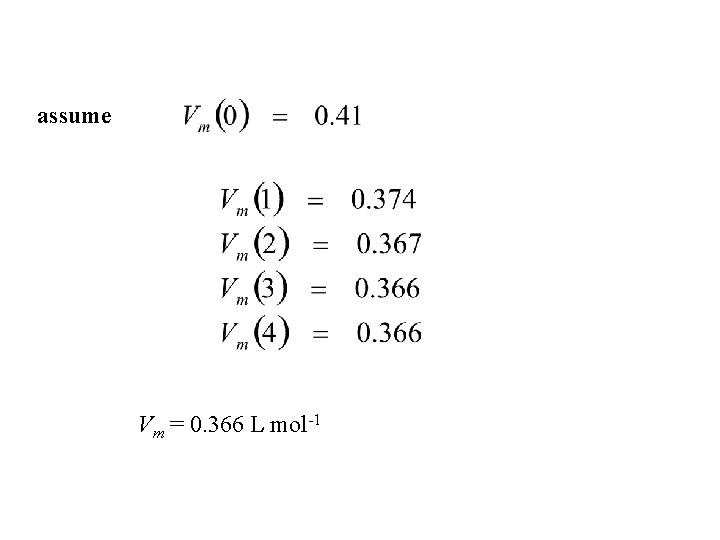
Example 2. 1 Estimate the molar volume of CO 2 at 500 K and 100 atm by treating it as a van der waals gas. From

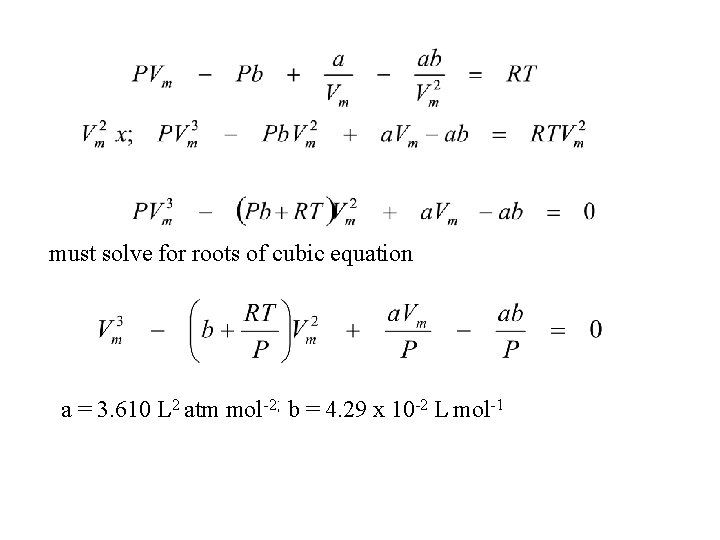
must solve for roots of cubic equation a = 3. 610 L 2 atm mol-2; b = 4. 29 x 10 -2 L mol-1
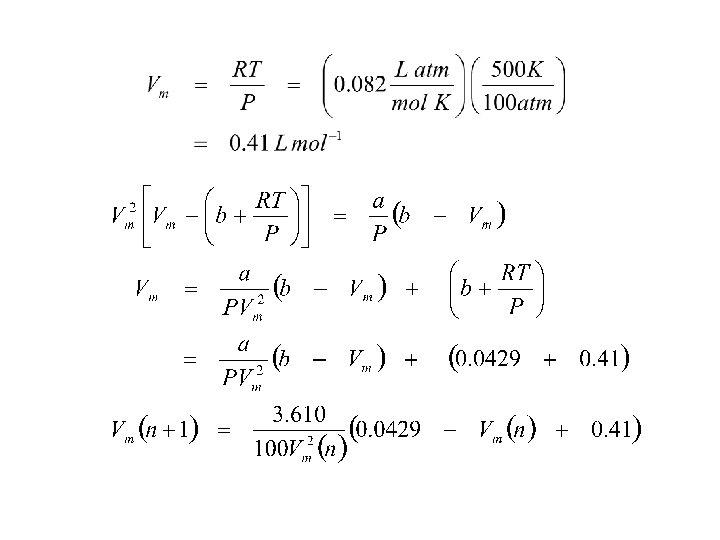

assume Vm = 0. 366 L mol-1
 Kinetic molecular theory
Kinetic molecular theory Particle theory evaporation
Particle theory evaporation Kinetic theory of gases
Kinetic theory of gases Postulates of kinetic theory of gas
Postulates of kinetic theory of gas Kinetic theory of gases
Kinetic theory of gases Postulates of kinetic theory of gases
Postulates of kinetic theory of gases Write postulates of kinetic theory of gases
Write postulates of kinetic theory of gases Kinetic theory
Kinetic theory Molecular theory of gases and liquids
Molecular theory of gases and liquids Teoria cinetica molecular
Teoria cinetica molecular Kinetic molecular theory of solids
Kinetic molecular theory of solids Kinetic molecular theory volume
Kinetic molecular theory volume Adhesive force
Adhesive force Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Kinetic molecular theory
Kinetic molecular theory Kinetic theory def
Kinetic theory def Theory vs hypothesis
Theory vs hypothesis Charles law in terms of kinetic molecular theory
Charles law in terms of kinetic molecular theory Kenitic molecular theory
Kenitic molecular theory Equation of state of real gas
Equation of state of real gas Kinetic molecular theory
Kinetic molecular theory Tenets of kinetic molecular theory
Tenets of kinetic molecular theory Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Solids liquids and gases section 2 properties of fluids
Solids liquids and gases section 2 properties of fluids Properties of solids liquids and gases with examples
Properties of solids liquids and gases with examples Properties of gas
Properties of gas Four properties of gas
Four properties of gas 5 properties of gases
5 properties of gases Properties of gases
Properties of gases Inert gases properties
Inert gases properties What are the different properties of gas?
What are the different properties of gas? Gases have low densities
Gases have low densities Chemical properties of noble gases
Chemical properties of noble gases