Gases Kinetic Molecular Theory of Gases A gas


























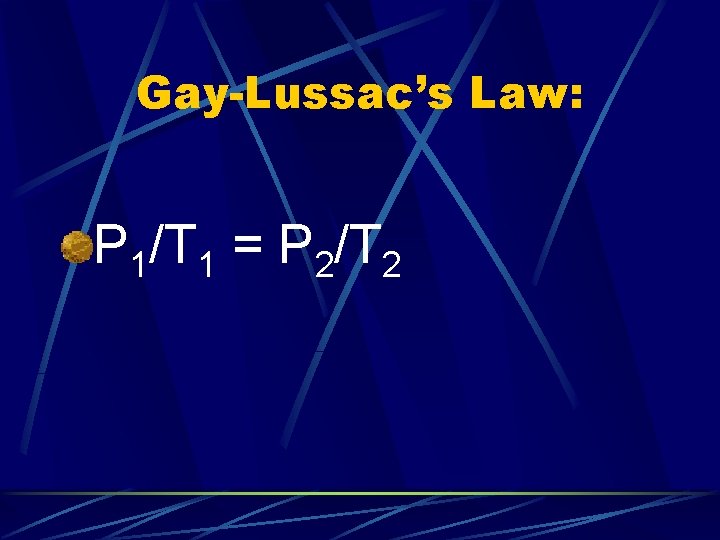

- Slides: 35

Gases Kinetic Molecular Theory of Gases

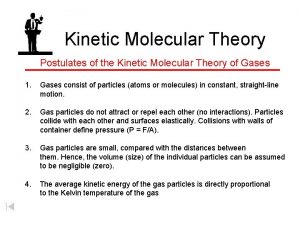
A gas consists of small particles (atoms/molecules) that move randomly with rapid velocities Further Information They move faster when heated.

The attractive forces between particles of a gas can be neglected Do you think this is accurate? Why would this be important for calculations?

The actual volume occupied by a gas molecule is extremely small compared to the volume that gas occupies. Is this true in the real world? Why would this be helpful with calculations?

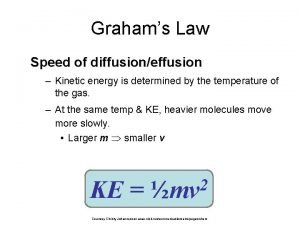
The average kinetic energy of a gas molecule is proportional to Kelvin temperature What is kinetic energy? Why Kelvin temperature and not Celsius or Fahrenheit? What does proportional mean?

Gas particles are in constant motion, moving rapidly in straight paths. * Is this true? What do we know about their motion? Why would the real situation make the calculations more difficult?

Ideal Gases An imaginary gas that perfectly fits all the assumptions of the kinetic molecular theory (KMT).

Expansion Gases do not have definite shape or volume. The expand to any container they are enclosed in. A gas in a 1 L container is then put into a 2 L container. How much volume does it have now?

Fluidity In an ideal gas, the gas particles glide past each other. This feature allows gases to be referred to as fluids just like liquids.

Low Density of a gas substance is only about 1/1000 of the same substance in liquid or solid state. Why is this true?

Compressibility This is a crowding effect of gases when the volume is decreased

Diffusion Spontaneous (does not require energy) mixing of particles of two substances caused by their random motion

Properties of a Gas Units of Measure

Pressure is not the same as force. Pressure is a force over an area. Example: psi = Pounds per in 2

Measuring Pressure A barometer measures atmospheric pressure.

Units of Pressure k. Pa, atm, mm of Hg, torr Helpful Conversions 1 atm = 760 mm Hg 1 atm = 760 torr 1 mm Hg = 1 torr 1 atm = 101. 325 k. Pa

Volume L, m. L or cm 3 Helpful conversions 1000 m. L = 1 L l 1 m. L = 1 cm 3 l

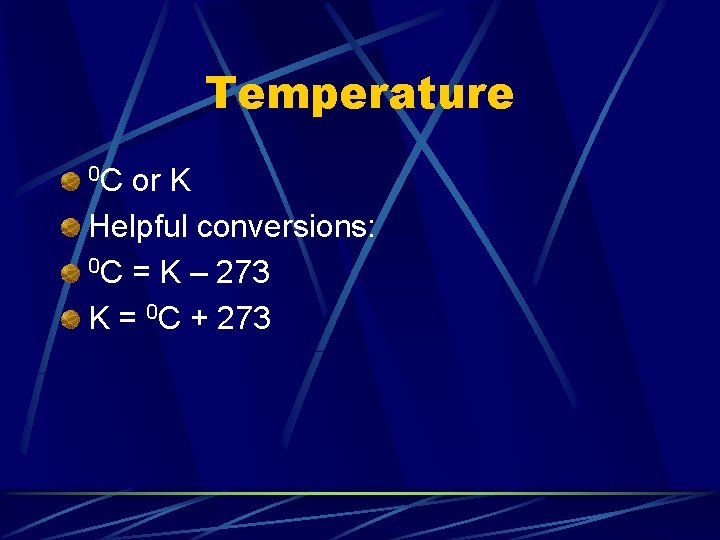
Temperature 0 C or K Helpful conversions: 0 C = K – 273 K = 0 C + 273

moles Number of moles = n If you are given grams, how would you convert to moles?

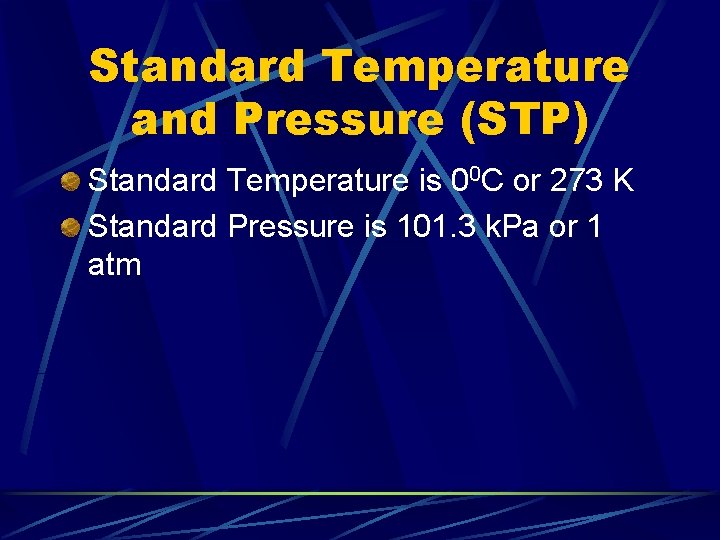
Standard Temperature and Pressure (STP) Standard Temperature is 00 C or 273 K Standard Pressure is 101. 3 k. Pa or 1 atm

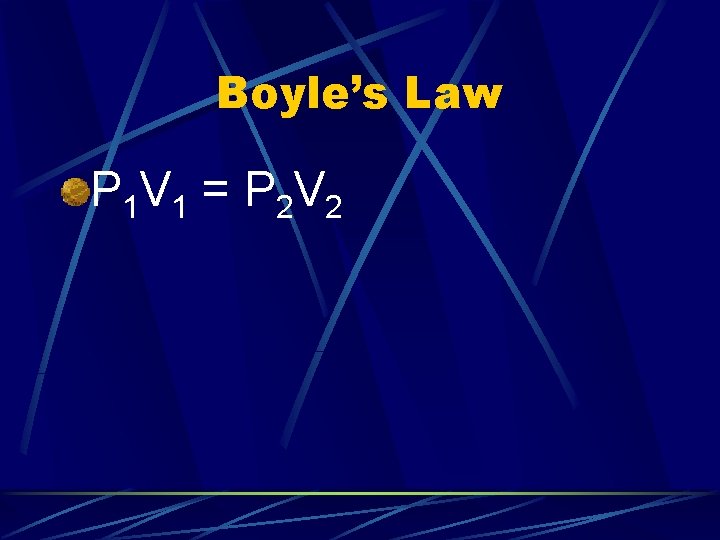
Boyle’s Law: Pressure and volume are inversely proportional P 1 V 1 = P 2 V 2

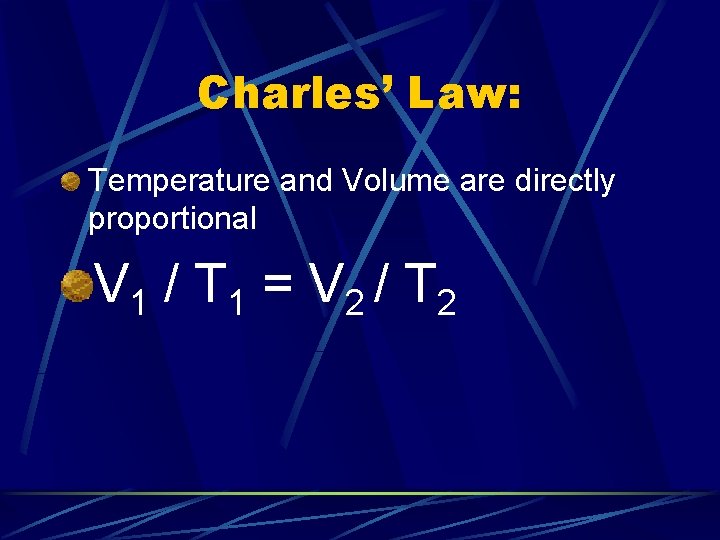
Charles’ Law: Temperature and Volume are directly proportional V 1 / T 1 = V 2 / T 2

Gay-Lussac’s Law: Pressure and Temperature are directly proportional P 1/T 1 = P 2/T 2

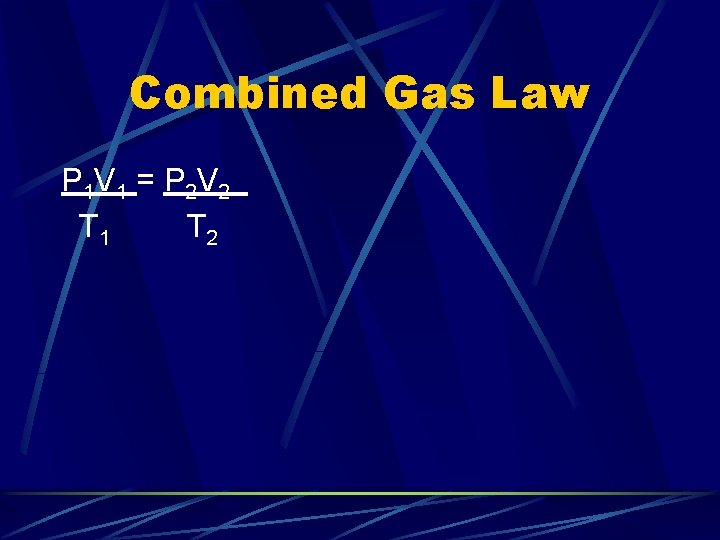
Combined Gas Law P 1 V 1 = P 2 V 2 T 1 T 2 If you remember this law, hold constant the other variables not used and you have all the gas laws we’ve used so far.

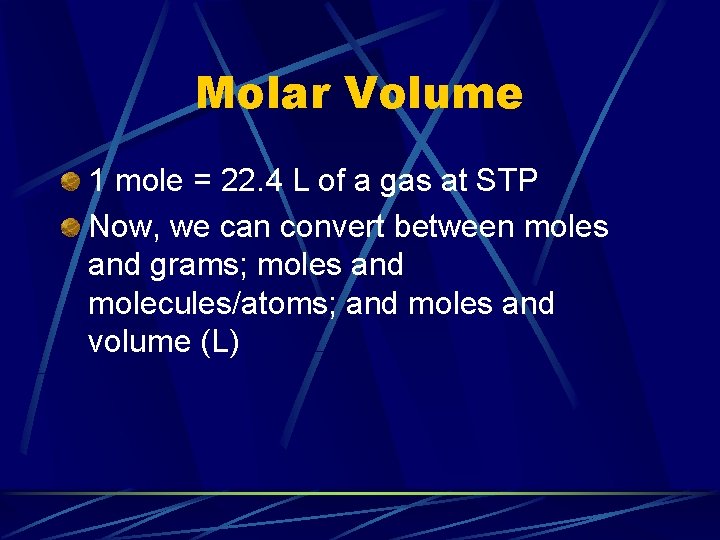
Molar Volume 1 mole = 22. 4 L of a gas at STP Now, we can convert between moles and grams; moles and molecules/atoms; and moles and volume (L)

Dalton’s Law of Partial Pressure The total pressure is equal to the sum of the partial pressures

Avagadro’s Law: V 1 / n 1 = V 2/n 2 Where n = number of moles How do you convert grams to moles?

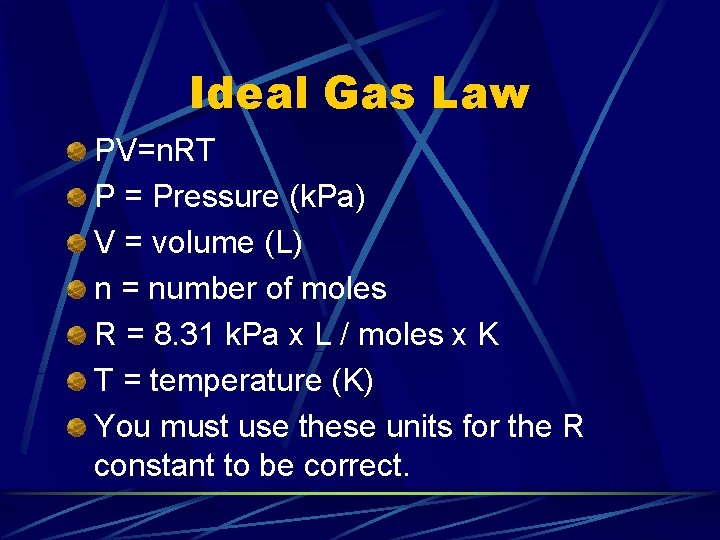
Ideal Gas Law PV=n. RT P = Pressure (k. Pa) V = volume (L) n = number of moles R = 8. 31 k. Pa x L / moles x K T = temperature (K) You must use these units for the R constant to be correct.

Name the Law! You will be given a series of laws and asked to name the law or you will be given the name and be asked to come up with the formula!

PV =n. RT Ideal Gas Law

V 1 / T 1 = V 2 / T 2 Charles’ Law

Boyle’s Law P 1 V 1 = P 2 V 2

Combined Gas Law P 1 V 1 = P 2 V 2 T 1 T 2
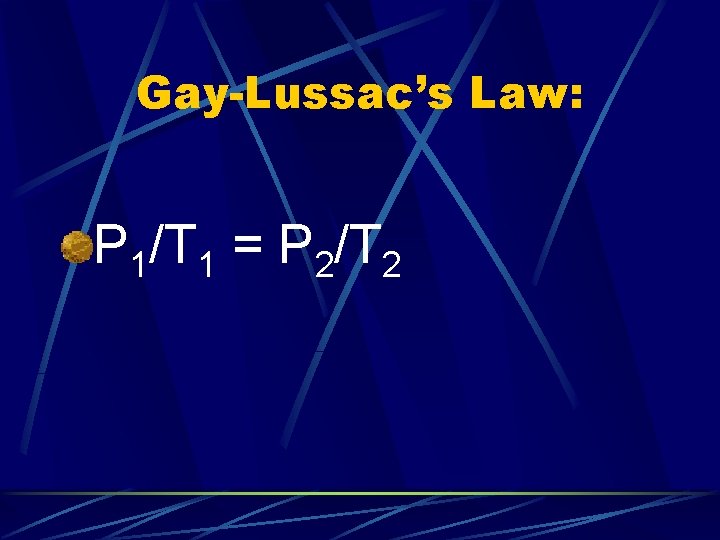
Gay-Lussac’s Law: P 1/T 1 = P 2/T 2

Avagadro’s Law: V 1 / n 1 = V 2/n 2
 Kinetic molecular model of gases
Kinetic molecular model of gases Kinetic molecular theory of solid
Kinetic molecular theory of solid Kinetic molecular theory volume
Kinetic molecular theory volume Adhesive force
Adhesive force Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Postulates of kinetic molecular theory
Postulates of kinetic molecular theory Kinetic molecular theory def
Kinetic molecular theory def Kinetic molecular theory timeline
Kinetic molecular theory timeline Charles law in terms of kinetic molecular theory
Charles law in terms of kinetic molecular theory Postulates of kinetic theory of gases class 11
Postulates of kinetic theory of gases class 11 Ideal gas law examples
Ideal gas law examples Write postulates of kinetic theory of gases
Write postulates of kinetic theory of gases Pv=1/3nmc^2
Pv=1/3nmc^2 Kinetic molecular theory
Kinetic molecular theory Tenets of kinetic molecular theory
Tenets of kinetic molecular theory Particle theory evaporation
Particle theory evaporation Kinetic theory of gases
Kinetic theory of gases Kinetic theory
Kinetic theory Postulates of kinetic theory of gases
Postulates of kinetic theory of gases Kinetic theory of gases
Kinetic theory of gases Molecular theory of gases and liquids
Molecular theory of gases and liquids Valence bond theory and molecular orbital theory
Valence bond theory and molecular orbital theory Valence bond vs molecular orbital theory
Valence bond vs molecular orbital theory Valence bond theory and molecular orbital theory
Valence bond theory and molecular orbital theory 11:55
11:55 Valence bond theory hybridization
Valence bond theory hybridization Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Zinc oxide + nitric acid → zinc nitrate + water
Zinc oxide + nitric acid → zinc nitrate + water Teoria cinetica molecular
Teoria cinetica molecular Relation between pressure and kinetic energy of gas
Relation between pressure and kinetic energy of gas Kinetic energy of gas molecules
Kinetic energy of gas molecules Graham's law of diffusion equation
Graham's law of diffusion equation The kinetic theory of matter states that
The kinetic theory of matter states that Kinetic theory of matter definition
Kinetic theory of matter definition Define kinetic theory of matter
Define kinetic theory of matter