Reaction Kinetics The Kinetic Theory of Matter states








- Slides: 39

Reaction Kinetics

The Kinetic Theory of Matter states that matter is composed of a large number a small particles—individual atoms or molecules— that are in constant motion. This constant motion is the reason that chemical reactions occur.

Kinetics � Branch of chemistry that deals with how fast reactions occur, Rates of Reactions. NO(g) + F 2(g) 2 NOF(g)

The Reaction Process Reaction Mechanism – chemical reactions occur through a series of smaller (step-like) reactions called elementary steps. Step 1: NO(g) + F 2(g) NOF 2 (g) Step 2: NOF 2 (g) + NO(g) 2 NOF(g) Overall Reaction: NO(g) + F 2(g) 2 NOF(g)

The Reaction Process Intermediates – products in one step that become reactants in a subsequent step (NOT Part of the overall reaction) Catalysts – substances that appear as a reactant in one step and as a product in a subsequent step. (NOT part of the overall reaction). Catalysts are used to INCREASE the rate of a reaction but they do not affect the outcome of the reaction.

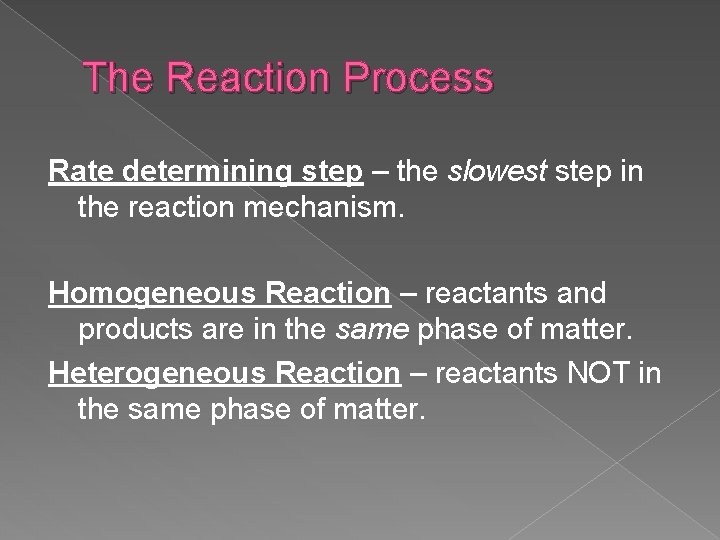
The Reaction Process Rate determining step – the slowest step in the reaction mechanism. Homogeneous Reaction – reactants and products are in the same phase of matter. Heterogeneous Reaction – reactants NOT in the same phase of matter.

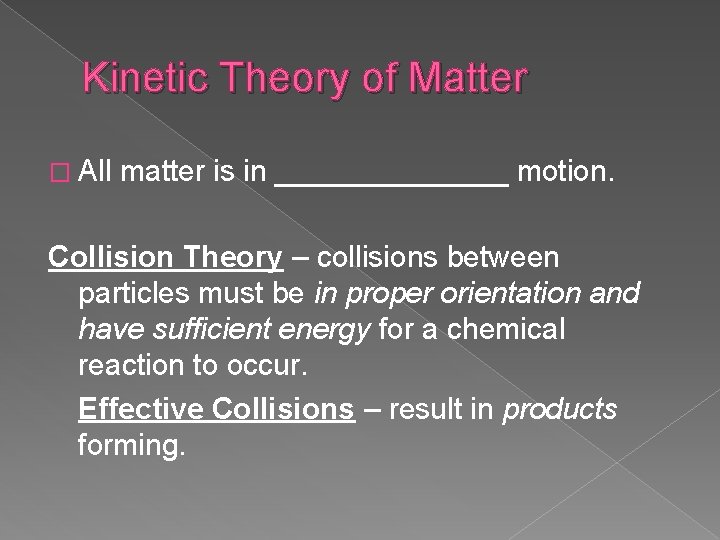
Kinetic Theory of Matter � All matter is in _______ motion. Collision Theory – collisions between particles must be in proper orientation and have sufficient energy for a chemical reaction to occur. Effective Collisions – result in products forming.

What are effective Collisions? Collisions with proper orientation. B) Collisions with sufficient energy. A) If A and B are not met ________!!!!

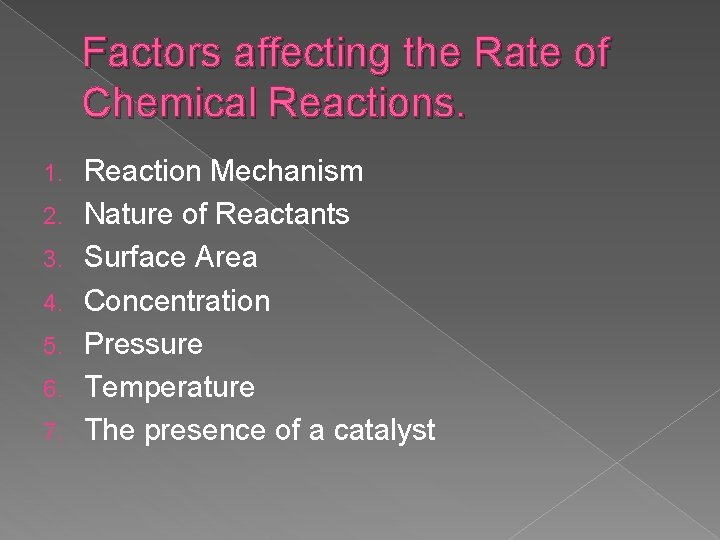
Factors affecting the Rate of Chemical Reactions. 1. 2. 3. 4. 5. 6. 7. Reaction Mechanism Nature of Reactants Surface Area Concentration Pressure Temperature The presence of a catalyst

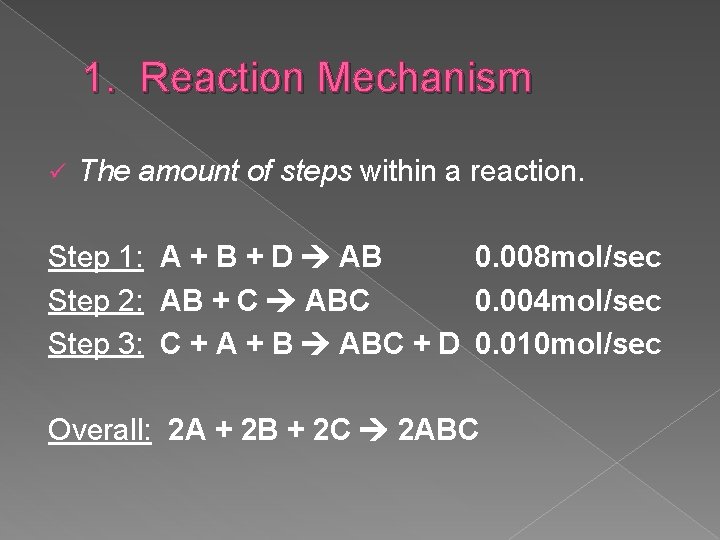
1. Reaction Mechanism ü The amount of steps within a reaction. Step 1: A + B + D AB 0. 008 mol/sec Step 2: AB + C ABC 0. 004 mol/sec Step 3: C + A + B ABC + D 0. 010 mol/sec Overall: 2 A + 2 B + 2 C 2 ABC

1. Reaction Mechanism � Which step was the rate determining step? �Step 2 at 0. 004 mol/sec (slowest step) � Which substance was the intermediate? �AB (products in one step that become reactants in a subsequent step, NOT part of the overall reaction) � Which substance was the catalyst? �D (appears as a reactant in one step and as a product in a subsequent step, NOT part of overall reaction. )

2. Nature of the Reactants ü Covalent Substances – react slowly due to a greater number of bonds that must be broken before the reaction can occur. ü Ionic Substances – react fast because ionic bonds are simply an attraction between positive and negative charges and no real bonds must to be broken.

2. Nature of Reactants � If ions are already present, they react the fastest!!! Example: Na. Cl(aq) vs. CH 4 Which one will react faster? ______

3. Surface Area The greater the surface area, the faster the reaction. ü The lesser the surface are, the slower the reaction. ü Ex) Steel wool rusts faster than a piece of steel. Ex)Wood chips burn faster than a log.

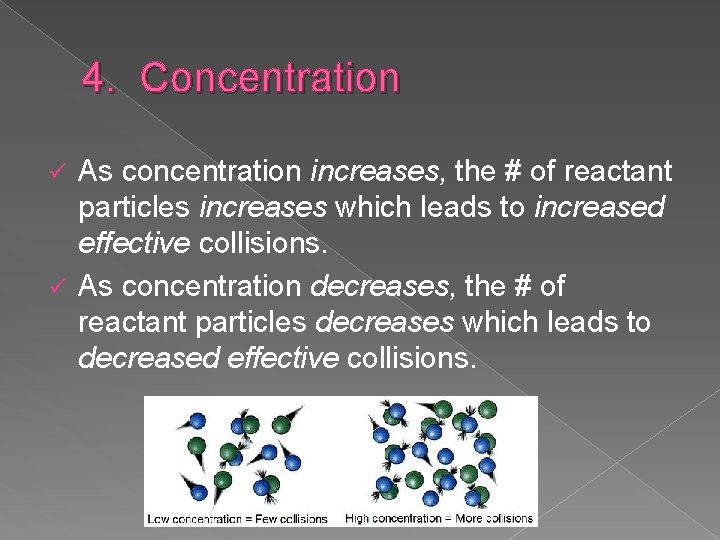
4. Concentration As concentration increases, the # of reactant particles increases which leads to increased effective collisions. ü As concentration decreases, the # of reactant particles decreases which leads to decreased effective collisions. ü

5. Pressure ü Only effect gases!!!! ü No effect on solids or liquids ü When pressure on a gas is applied, there is less room (volume) which will lead to more effective collisions.

6. Temperature ü Increased temperature leads to increased kinetic energy of particles, there more there is more chance for effective collisions to occur. üThe hotter something gets the faster its particles move.

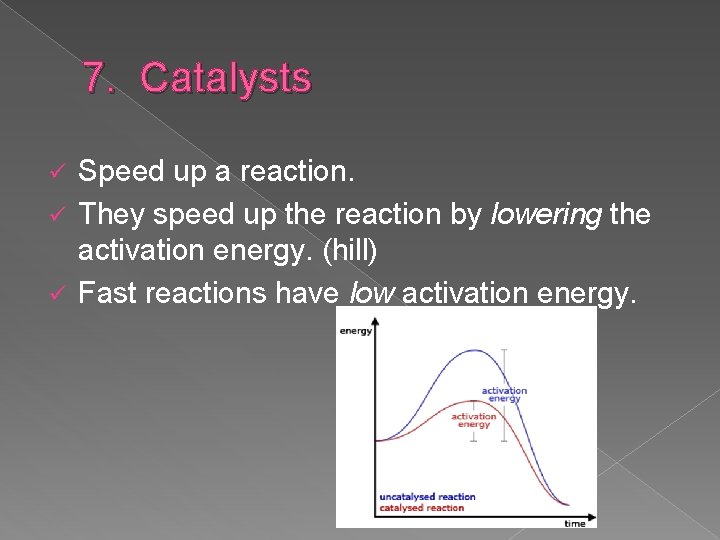
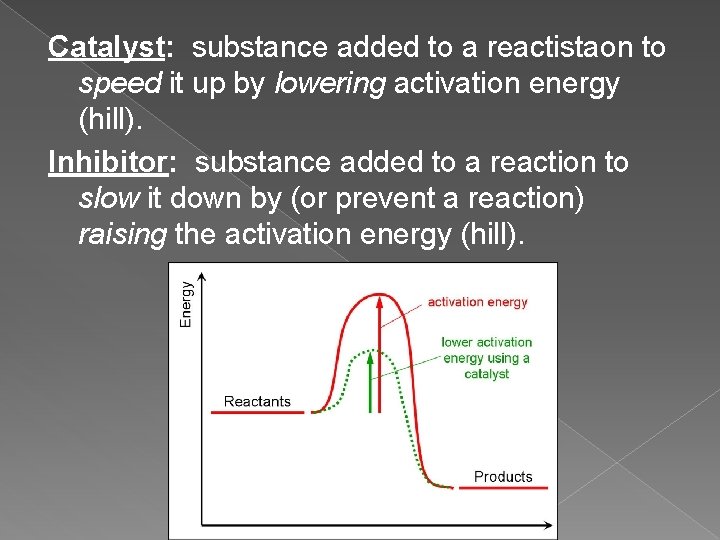
7. Catalysts Speed up a reaction. ü They speed up the reaction by lowering the activation energy. (hill) ü Fast reactions have low activation energy. ü

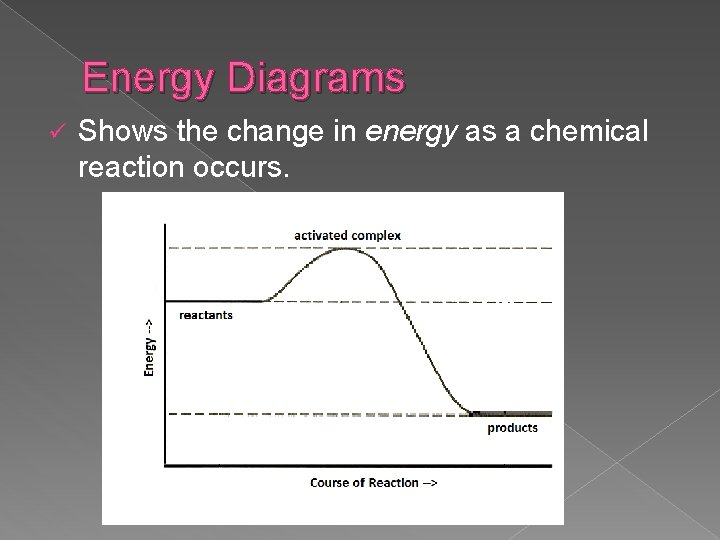
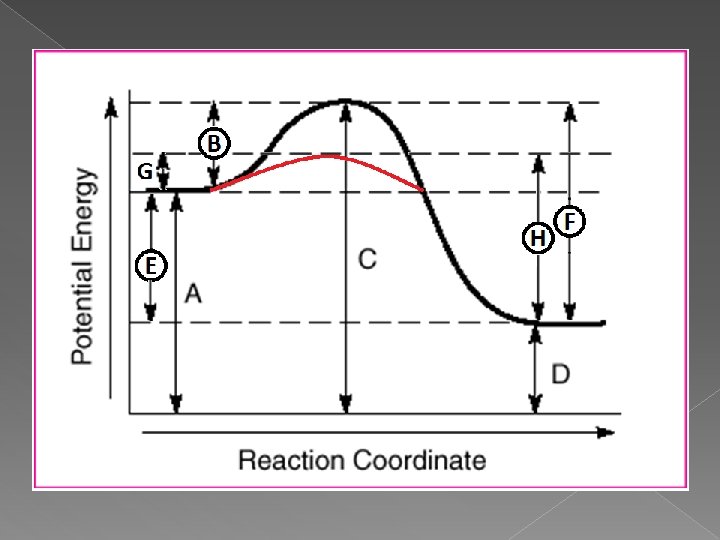
Energy Diagrams ü Shows the change in energy as a chemical reaction occurs.

Vocabulary associated with energy diagrams Activation Energy: energy needed to change reactants into products. (Energy needed to go up the hill. ) Activated Complex: a temporary, intermediate product that either re-forms into reactants or forms new products. (Point at the top of the hill. ) Heat of Reaction (∆H): heat energy emitted or absorbed when products form. ∆H = Hproducts - Hreactants


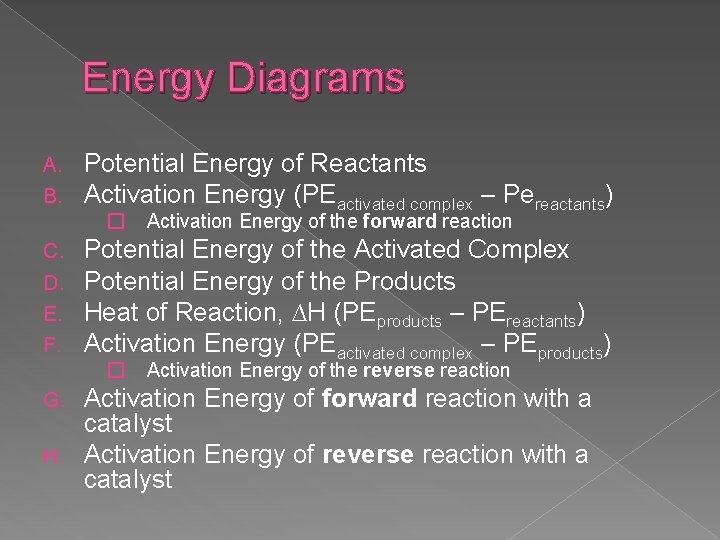
Energy Diagrams A. B. Potential Energy of Reactants Activation Energy (PEactivated complex – Pereactants) � Activation Energy of the forward reaction C. D. E. F. Potential Energy of the Activated Complex Potential Energy of the Products Heat of Reaction, ∆H (PEproducts – PEreactants) Activation Energy (PEactivated complex – PEproducts) � Activation Energy of the reverse reaction Activation Energy of forward reaction with a catalyst H. Activation Energy of reverse reaction with a catalyst G.

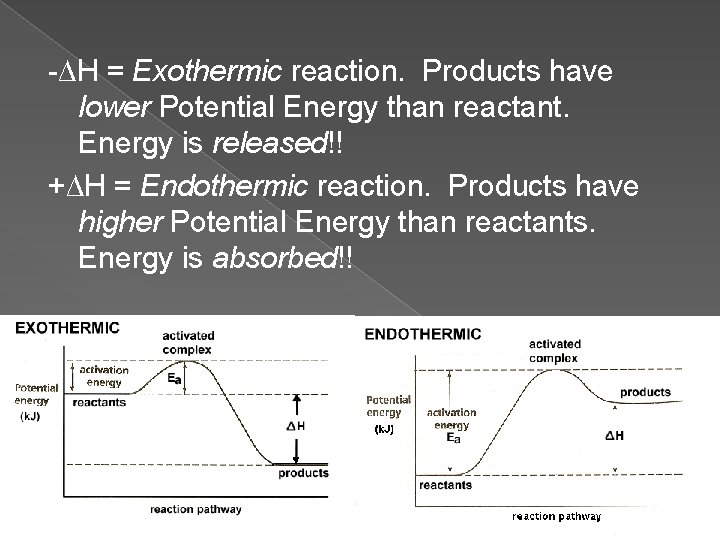
-∆H = Exothermic reaction. Products have lower Potential Energy than reactant. Energy is released!! +∆H = Endothermic reaction. Products have higher Potential Energy than reactants. Energy is absorbed!!

Catalyst: substance added to a reactistaon to speed it up by lowering activation energy (hill). Inhibitor: substance added to a reaction to slow it down by (or prevent a reaction) raising the activation energy (hill).

The Two Fundamental Drives in Nature 1) Enthalpy (∆H): Tendency in nature toward a lower energy state. ü Nature favors loss of heat (lower enthalpy) ü Exothermic reactions are favored ü The activation energy for exothermic reactions less than endothermic reactions. This makes it more likely for exothermic reactions to have enough activation energy to be successful.

The Two Fundamental Drives in Nature 2) Entropy (∆S): The tendency of nature toward a state of randomness, disorder, chaos. Tidy room, Low Entropy Messy room, High Entropy

Entropy ü ü ü The greater the disorder, the higher the entropy. Nature favors higher entropy. As temperature increases, entropy increases Low entropy = more order High entropy = more chaos, disorder, randomness.

The Two Fundamental Drives in Nature Toward lower enthalpy (heat) (-∆H) 2. Toward greater entropy (randomness)(+∆S) 1. A spontaneous reaction occurs if the 2 drives are met. If the 2 drives are not met, the reaction does NOT occur.

Free Energy � Allows one to predict if a reaction will be spontaneous. Formula: ∆G = ∆H - T∆S ∆G = (Gibb’s) change in free energy ∆H = heat of reaction enthalpy T = temperature in Kelvin ∆S = change in entropy The sign of ∆G (+/-) will decide whether or not a reaction is spontaneous when only 1 of the 2 drives is favored.

Table G � Lists the free energy of formation and heat of formation for many compounds. At 298 K, which substance on Table G has an endothermic heat of reaction but will form spontaneously from its elements? Spontaneous: -∆G Non-Spontaneous: +∆G Equilibrium: ∆G = 0

Thermochemical Equations Energy is stored in chemical bonds! Heat of Reaction: ∆H = Hp – Hr (change of energy – heat of a chemical reaction) ü Matter always tries to reach lower potential energy. ü Nature favors exothermic reactions. ü Low enthalpy (low energy) = increased stability. Thermochemical Equation: an equation that indicates the energy change (∆H). The phases of all substances will be shown.

Types of Reactions 1. Endothermic: heat is absorbed. ü ü ü Heat is shown as a reactant. Heat of the reactants is lower than the heat of the products. ∆H is positive. Enthalpy is high in these reactions. These reactions are NOT favored in nature. Ex) 2 H 20(l) + 571. 6 k. J 2 H 2(g) + O 2(g) Question: If 4 moles of H 2 O(l) decompose, how much heat is absorbed?

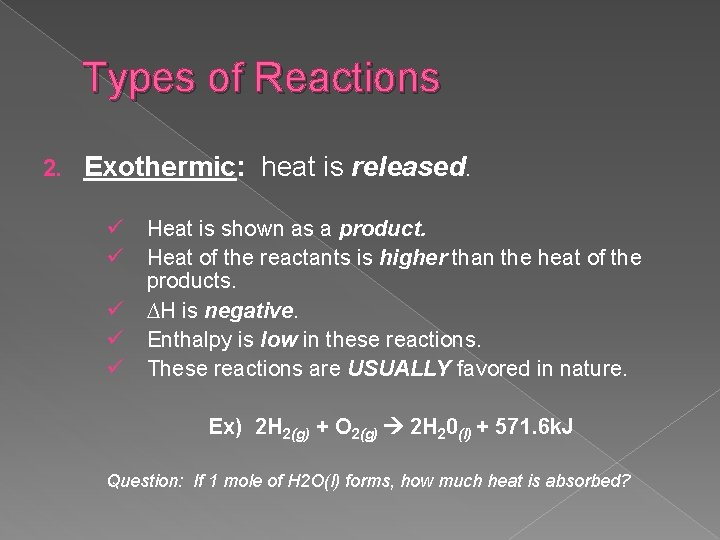
Types of Reactions 2. Exothermic: heat is released. ü ü ü Heat is shown as a product. Heat of the reactants is higher than the heat of the products. ∆H is negative. Enthalpy is low in these reactions. These reactions are USUALLY favored in nature. Ex) 2 H 2(g) + O 2(g) 2 H 20(l) + 571. 6 k. J Question: If 1 mole of H 2 O(l) forms, how much heat is absorbed?

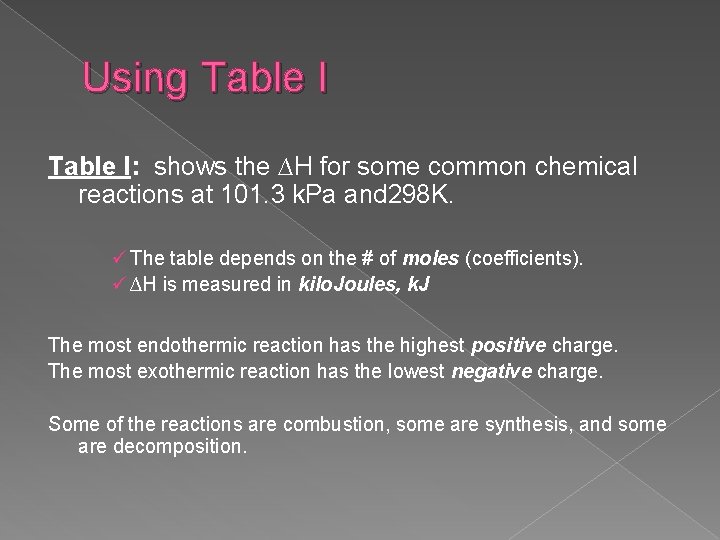
Using Table I: shows the ∆H for some common chemical reactions at 101. 3 k. Pa and 298 K. ü The table depends on the # of moles (coefficients). ü ∆H is measured in kilo. Joules, k. J The most endothermic reaction has the highest positive charge. The most exothermic reaction has the lowest negative charge. Some of the reactions are combustion, some are synthesis, and some are decomposition.

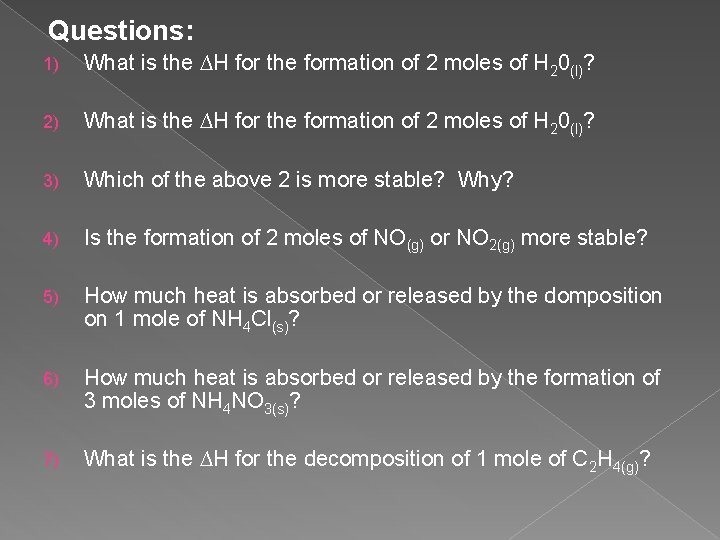
Questions: 1) What is the ∆H for the formation of 2 moles of H 20(l)? 2) What is the ∆H for the formation of 2 moles of H 20(l)? 3) Which of the above 2 is more stable? Why? 4) Is the formation of 2 moles of NO(g) or NO 2(g) more stable? 5) How much heat is absorbed or released by the domposition on 1 mole of NH 4 Cl(s)? 6) How much heat is absorbed or released by the formation of 3 moles of NH 4 NO 3(s)? 7) What is the ∆H for the decomposition of 1 mole of C 2 H 4(g)?

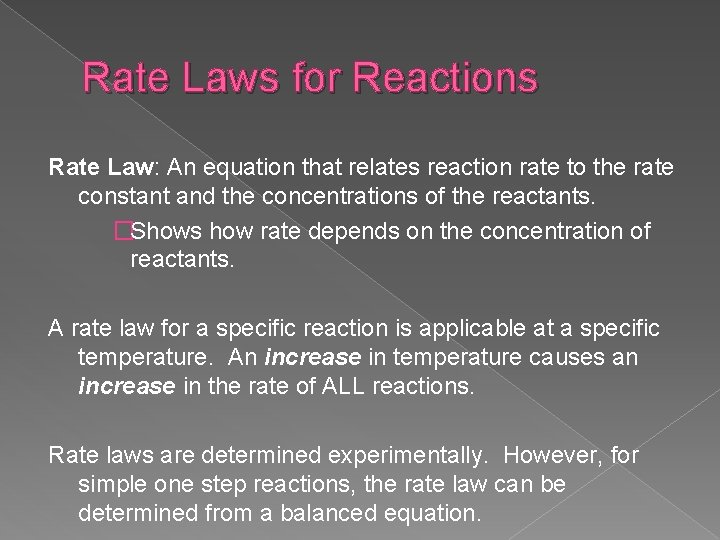
Rate Laws for Reactions Rate Law: An equation that relates reaction rate to the rate constant and the concentrations of the reactants. �Shows how rate depends on the concentration of reactants. A rate law for a specific reaction is applicable at a specific temperature. An increase in temperature causes an increase in the rate of ALL reactions. Rate laws are determined experimentally. However, for simple one step reactions, the rate law can be determined from a balanced equation.

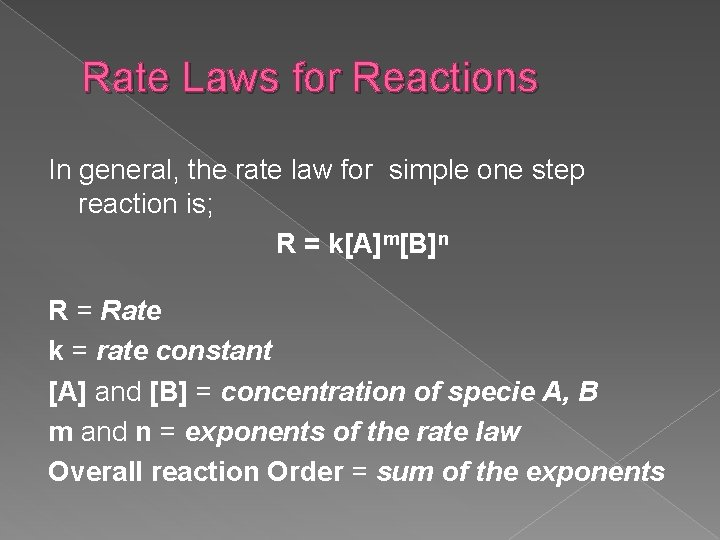
Rate Laws for Reactions In general, the rate law for simple one step reaction is; R = k[A]m[B]n R = Rate k = rate constant [A] and [B] = concentration of specie A, B m and n = exponents of the rate law Overall reaction Order = sum of the exponents

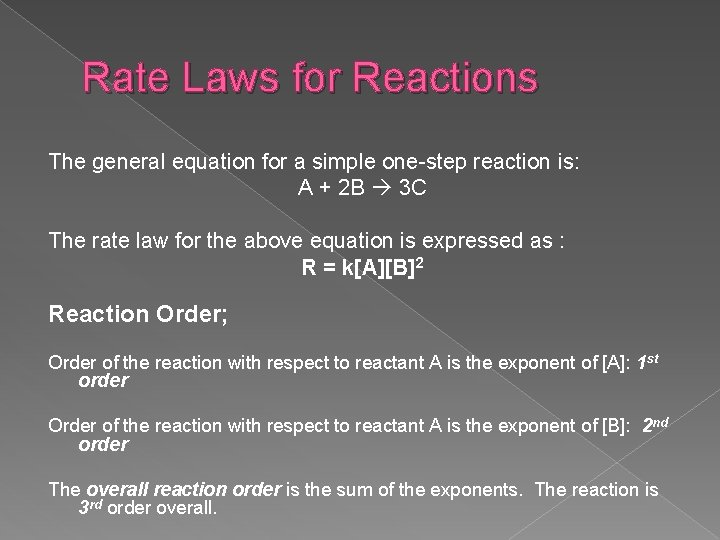
Rate Laws for Reactions The general equation for a simple one-step reaction is: A + 2 B 3 C The rate law for the above equation is expressed as : R = k[A][B]2 Reaction Order; Order of the reaction with respect to reactant A is the exponent of [A]: 1 st order Order of the reaction with respect to reactant A is the exponent of [B]: 2 nd order The overall reaction order is the sum of the exponents. The reaction is 3 rd order overall.

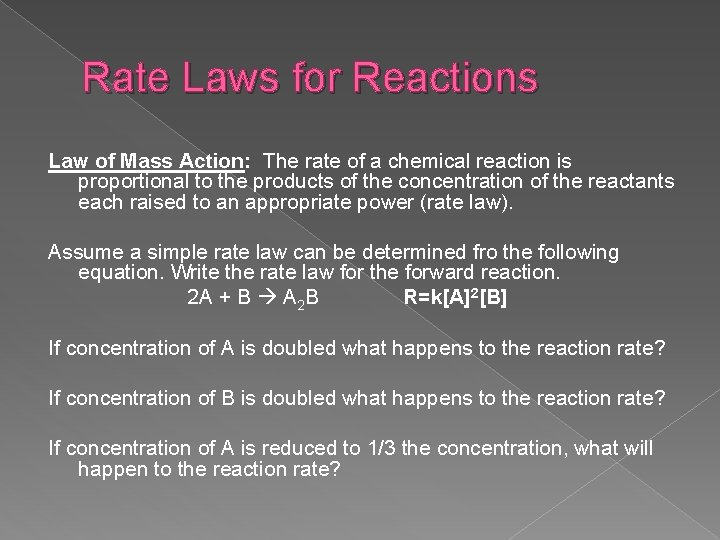
Rate Laws for Reactions Law of Mass Action: The rate of a chemical reaction is proportional to the products of the concentration of the reactants each raised to an appropriate power (rate law). Assume a simple rate law can be determined fro the following equation. Write the rate law for the forward reaction. 2 A + B A 2 B R=k[A]2[B] If concentration of A is doubled what happens to the reaction rate? If concentration of B is doubled what happens to the reaction rate? If concentration of A is reduced to 1/3 the concentration, what will happen to the reaction rate?