Kinetic Theory Kinetic Theory Kinetic refers to movement































- Slides: 45

Kinetic Theory

Kinetic Theory • “Kinetic” refers to movement

Kinetic Theory • “Kinetic” refers to movement • “Theory, ” a unifying idea that is useful for understanding a wide variety of observations

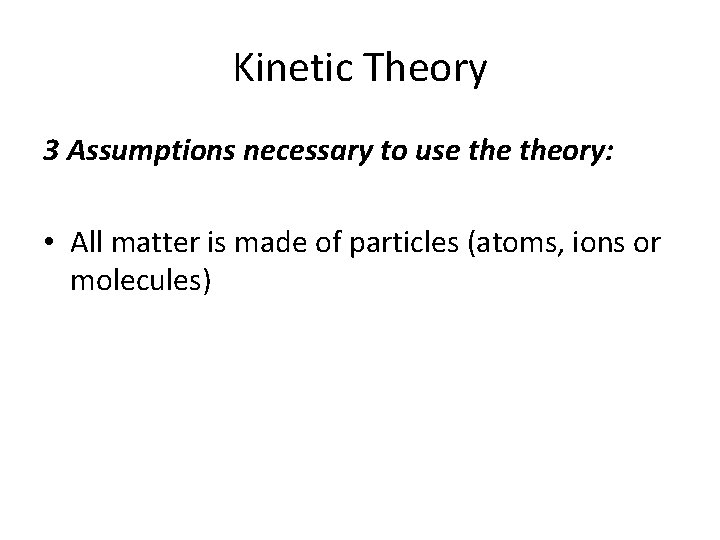
Kinetic Theory • The kinetic theory states that submicroscopic particles of all matter are in constant, random motion. • The Kinetic Theory is a unifying concept that uses the idea of moving particles to explain gases, liquids, solids, phase changes and the effect of temperature and pressure on these.

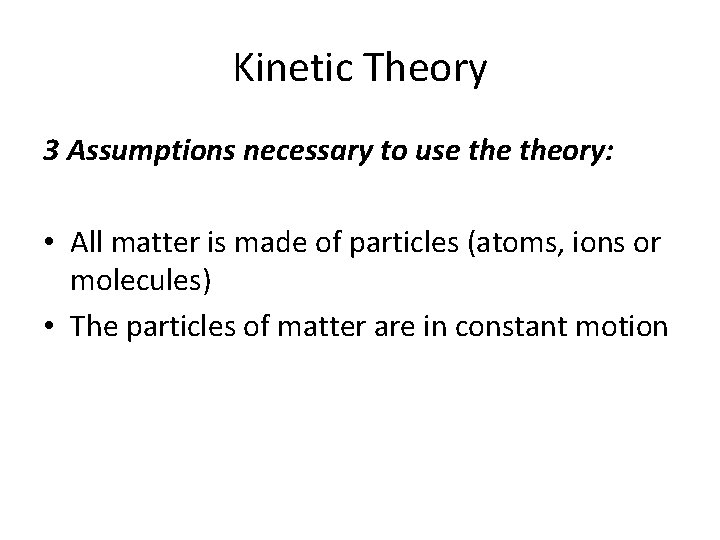
Kinetic Theory 3 Assumptions necessary to use theory:

Kinetic Theory 3 Assumptions necessary to use theory: • All matter is made of particles (atoms, ions or molecules)

Kinetic Theory 3 Assumptions necessary to use theory: • All matter is made of particles (atoms, ions or molecules) • The particles of matter are in constant motion

Kinetic Theory 3 Assumptions necessary to use theory: • All matter is made of particles (atoms, ions or molecules) • The particles of matter are in constant motion • All collisions are perfectly elastic (no energy is lost)

Solid

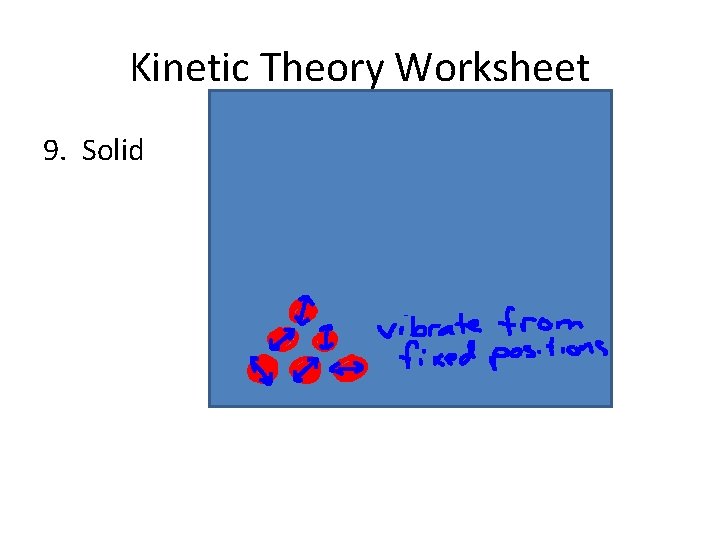
Solid • Solids have their own shape and volume regardless of their containers.

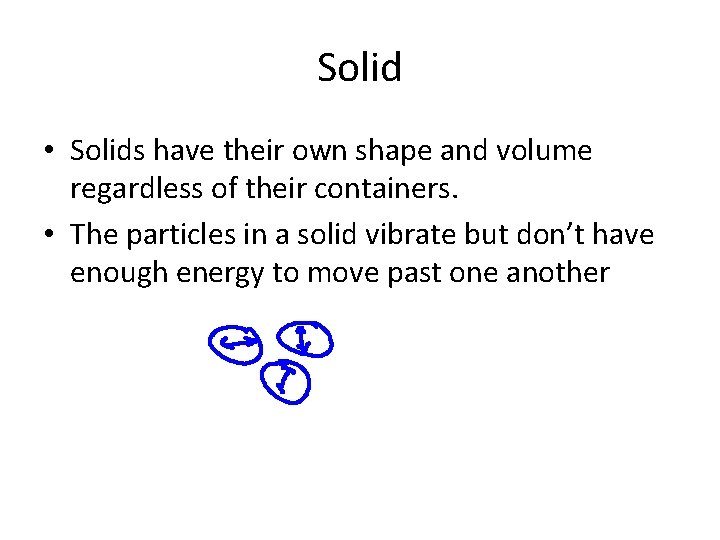
Solid • Solids have their own shape and volume regardless of their containers. • The particles in a solid vibrate but don’t have enough energy to move past one another

Solid • Solids have their own shape and volume regardless of their containers. • The particles in a solid vibrate but don’t have enough energy to move past one another • But, if you heat a solid (giving it more energy) it will ______ and become a ….

Liquid

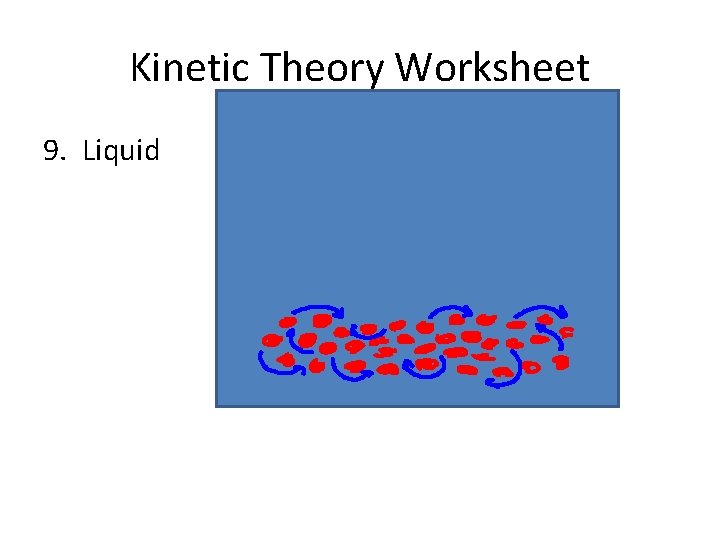
Liquid • The particles in a liquid have enough energy to flow past one another, and thus take the shape of whatever container they are in.

Liquid • The particles in a liquid have enough energy to flow past one another, and thus take the shape of whatever container they are in. • But they don’t have enough energy to break the attractive forces between them so they remain in contact and maintain their own volume.

Liquid • The particles in a liquid have enough energy to flow past one another, and thus take the shape of whatever container they are in. • But they don’t have enough energy to break the attractive forces between them so they remain in contact and maintain their own volume. • If you heat a liquid (giving it more energy) it will _____, and become a …

Gas

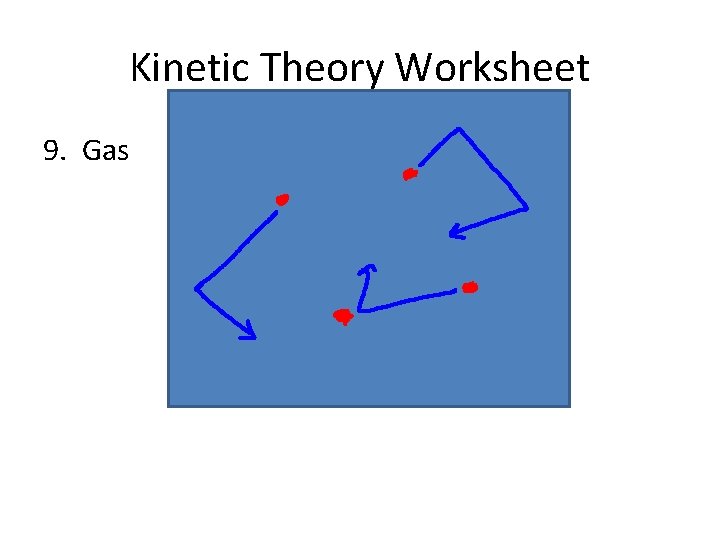
Gas • The particles of a gas have enough energy to overwhelm any attractive forces between them. If they collide, they bounce off without losing energy.

Gas • The particles of a gas have enough energy to overwhelm any attractive forces between them. If they collide, they bounce off without losing energy. • Gases take the shape and volume of their containers.

Gas • The particles of a gas have enough energy to overwhelm any attractive forces between them. If they collide, they bounce off without losing energy. • Gases take the shape and volume of their containers. • If you cool a gas, it _____ and becomes a…

Liquid • If you cool a liquid, the particles will lose energy and will _____ to become a …

Solid • If you cool a solid, it will lose energy and vibrate more and more slowly until a temperature is reached at which no movement occurs.

Solid • If you cool a solid, it will lose energy and vibrate more and more slowly until a temperature is reached at which no movement occurs. • This temperature is called ABSOLUTE ZERO, and is theoretically the coldest possible temperature.

Temperature • We will soon be defining temperature as a measure of the average kinetic energy of a substance.

Simulation • (hit escape then double click the link below)

Kinetic Theory Worksheet 1. What is the main idea of the kinetic theory?

Kinetic Theory Worksheet 1. What is the main idea of the kinetic theory? (the movement of particles causes many of the properties of matter that we observe)

Kinetic Theory Worksheet 2. What are three assumptions one must make to use theory?

Kinetic Theory Worksheet 2. What are three assumptions one must make to use theory? Matter is made of particles Particles are in constant motion Collisions are elastic

Kinetic Theory Worksheet 3. What are three most common states of matter on Earth?

Kinetic Theory Worksheet 3. What are three most common states of matter on Earth? Solid, liquid, gas

Kinetic Theory Worksheet 4. In which state(s) are the particles separated by much empty space?

Kinetic Theory Worksheet 4. In which state(s) are the particles separated by much empty space? gas

Kinetic Theory Worksheet 5. In which state(s) are the particles in contact with one another?

Kinetic Theory Worksheet 5. In which state(s) are the particles in contact with one another? Solid and liquid

Kinetic Theory Worksheet 6. In which state(s) are the particles in contact with one another but have enough kinetic energy to flow past one another?

Kinetic Theory Worksheet 6. In which state(s) are the particles in contact with one another but have enough kinetic energy to flow past one another? liquid

Kinetic Theory Worksheet 7. In which state(s) are the particles in contact with one another but don’t have enough kinetic energy to move past one another, but instead vibrate from their fixed positions?

Kinetic Theory Worksheet 7. In which state(s) are the particles in contact with one another but don’t have enough kinetic energy to move past one another, but instead vibrate from their fixed positions? solid

Kinetic Theory Worksheet 8. Which state would be most compressible? Why?

Kinetic Theory Worksheet 8. Which state would be most compressible? Why? Gas. Because there is empty space around the particles.

Kinetic Theory Worksheet 9. Gas

Kinetic Theory Worksheet 9. Liquid

Kinetic Theory Worksheet 9. Solid

 It is generally refers to human movement
It is generally refers to human movement Movement area
Movement area Or axial movement are done in place.
Or axial movement are done in place. Describe the cognitive theory of learning movement skills
Describe the cognitive theory of learning movement skills Revitalization movement definition
Revitalization movement definition Social movement adalah
Social movement adalah Kinetic molecular theory of solid
Kinetic molecular theory of solid The kinetic theory of matter states that
The kinetic theory of matter states that Kinetic theory of matter definition
Kinetic theory of matter definition Kinetic theory of matter
Kinetic theory of matter The kinetic theory explains how particles in matter behave
The kinetic theory explains how particles in matter behave Kinetic molecular theory
Kinetic molecular theory The kinetic molecular theory
The kinetic molecular theory Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Kinetic molecular theory
Kinetic molecular theory Kinetic molecular theory def
Kinetic molecular theory def Particle theory melting
Particle theory melting Timeline of kinetic molecular theory
Timeline of kinetic molecular theory The attraction between particles gives solids a definite
The attraction between particles gives solids a definite Kinetic theory of gases
Kinetic theory of gases Charles law in terms of kinetic molecular theory
Charles law in terms of kinetic molecular theory Three postulates of kinetic theory of gases
Three postulates of kinetic theory of gases Kenitic molecular theory
Kenitic molecular theory Kinetic theory of gases
Kinetic theory of gases What is a kinetic theory of matter
What is a kinetic theory of matter Write the postulates of kinetic theory of gases
Write the postulates of kinetic theory of gases Pv=1/3nmc^2
Pv=1/3nmc^2 Write the postulates of kinetic theory of gases
Write the postulates of kinetic theory of gases Kinetic molecular theory
Kinetic molecular theory General gas equation is
General gas equation is Tenets of kinetic molecular theory
Tenets of kinetic molecular theory Diversity refers to:
Diversity refers to: Corporate culture refers to
Corporate culture refers to Working capital management refers to
Working capital management refers to Text structure refers to
Text structure refers to What is text structure
What is text structure Scope refers to....
Scope refers to.... Variability statistics
Variability statistics Value added model education
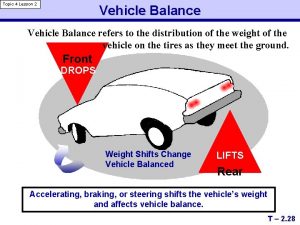
Value added model education What is vehicle balance
What is vehicle balance Limits the impact of vehicle balance
Limits the impact of vehicle balance Belief perseverance
Belief perseverance Rhythm by opposition fashion
Rhythm by opposition fashion Refers to the variety of training a performer undertake
Refers to the variety of training a performer undertake Fitt exam questions
Fitt exam questions