KINETIC THEORY Kinetic Theory states that the tiny








- Slides: 15

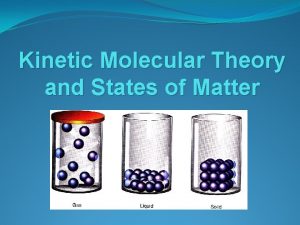
KINETIC THEORY § Kinetic Theory states that the tiny particles in all forms of matter are in constant motion. § Kinetic refers to motion § Helps you understand the behavior of solid, liquid, and gas atoms/molecules as well as the physical properties § Provides a model behavior based off three principals

KINETIC THEORY § 3 Principles of Kinetic Theory § All matter is made of tiny particles (atoms) § These particles are in constant motion § When particles collide with each other or the container, the collisions are perfectly elastic (no energy is lost)

SOLIDS § § § Particles are tightly packed and close together Particles do move but not very much Definite shape and definite volume (because particles are packed closely and do not move) § Most solids are crystals § Crystals are made of unit cells (repeating patterns) § The shape of a crystal reflects the arrangement of the particles within the solid

SOLIDS § Unit cells put together make a crystal lattice (skeleton for the crystal) § Crystals are classified into seven crystal systems: cubic, tetragonal, orthorhombic, monoclinic, triclinic, hexagonal, rhombohedral § Unit cell crystal lattice solid

SOLIDS § Amorphous Solid: § A solid with no defined shape (not a crystal) § A solid that lacks an ordered internal structure § Examples: Clay, Play. Doh, Rubber, Glass, Plastic, Asphalt § Allotropes: § Solids that appear in more than one form § 2 or more different molecular forms of the same element in the same physical state (have different properties) § Example: Carbon § § § Powder = Graphite Pencil “lead” = graphite Hard solid = diamond

SOLIDS www. ohsu. edu/research/sbh/resultsimages/crystalvsglass. gif

LIQUIDS § § § Particles are spread apart Particles move slowly through a container No definite shape but do have a definite volume Flow from one container to another Viscosity – resistance of a liquid to flowing § Honey – high viscosity § Water – low viscosity chemed. chem. purdue. edu/. . . /graphics

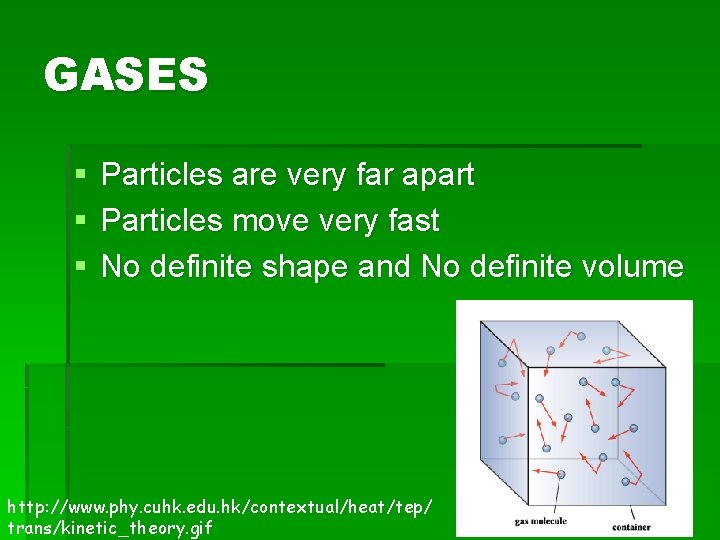
GASES § § § Particles are very far apart Particles move very fast No definite shape and No definite volume http: //www. phy. cuhk. edu. hk/contextual/heat/tep/ trans/kinetic_theory. gif

GASES AND PRESSURE § Gas pressure is the force exerted by a gas per unit surface area of an object § Force and number of collisions § When there are no particles present, no collisions = no pressure = vacuum § Standard Pressure is average normal pressure at sea level § As you go ABOVE sea level, pressure is less § As you go BELOW sea level, pressure is greater

GASES AND PRESSURE § Standard Pressure Values § At sea level the pressure can be recorded as: § § § 14. 7 psi (pounds per square inch) 29. 9 in. Hg (inches of Mercury) 760 mm. Hg (millimeters of Mercury) 760 torr 1 atm (atmosphere) 101. 325 k. Pa (kilopascals) § All of these values are EQUAL to each other: § § 29. 9 in. Hg = 101. 325 k. Pa 760 torr = 760 mm. Hg 1 atm = 14. 7 psi and so on………. § Say hello to Factor Label Method!!!!!!

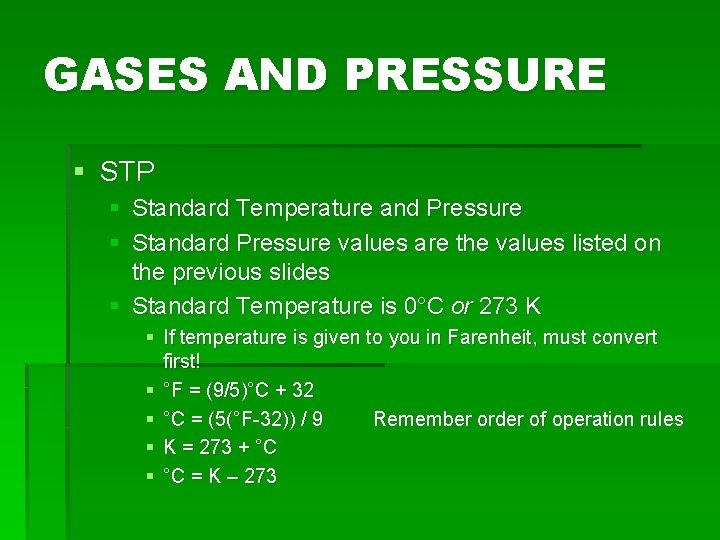
GASES AND PRESSURE § STP § Standard Temperature and Pressure § Standard Pressure values are the values listed on the previous slides § Standard Temperature is 0°C or 273 K § If temperature is given to you in Farenheit, must convert first! § °F = (9/5)°C + 32 § °C = (5(°F-32)) / 9 Remember order of operation rules § K = 273 + °C § °C = K – 273

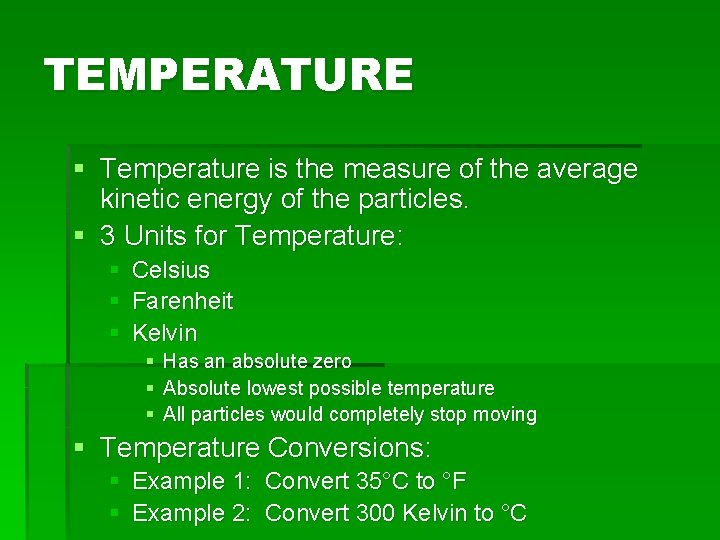
TEMPERATURE § Temperature is the measure of the average kinetic energy of the particles. § 3 Units for Temperature: § Celsius § Farenheit § Kelvin § Has an absolute zero § Absolute lowest possible temperature § All particles would completely stop moving § Temperature Conversions: § Example 1: Convert 35°C to °F § Example 2: Convert 300 Kelvin to °C

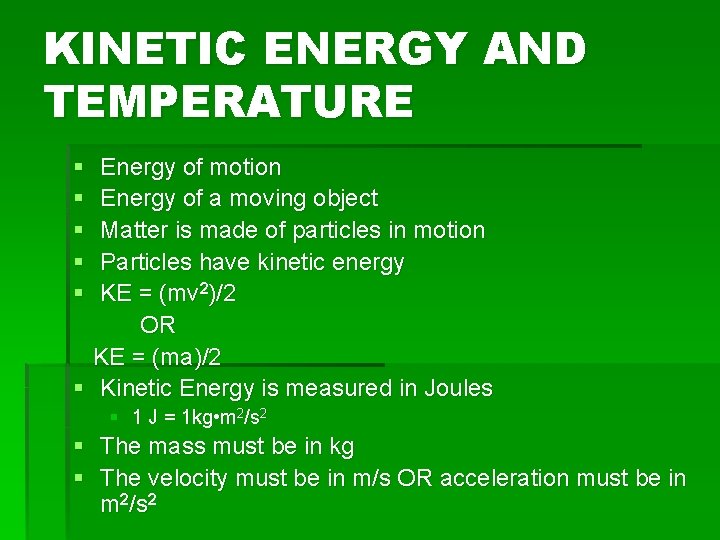
KINETIC ENERGY AND TEMPERATURE § § § Energy of motion Energy of a moving object Matter is made of particles in motion Particles have kinetic energy KE = (mv 2)/2 OR KE = (ma)/2 § Kinetic Energy is measured in Joules § 1 J = 1 kg • m 2/s 2 § The mass must be in kg § The velocity must be in m/s OR acceleration must be in m 2/s 2

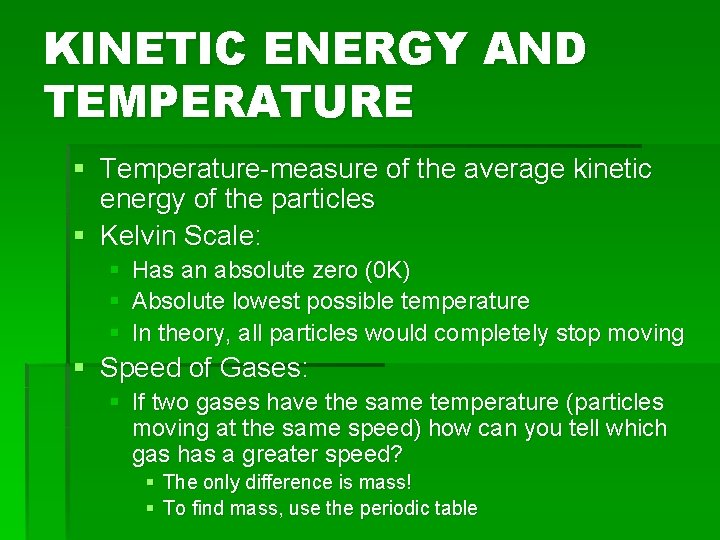
KINETIC ENERGY AND TEMPERATURE § Temperature-measure of the average kinetic energy of the particles § Kelvin Scale: § Has an absolute zero (0 K) § Absolute lowest possible temperature § In theory, all particles would completely stop moving § Speed of Gases: § If two gases have the same temperature (particles moving at the same speed) how can you tell which gas has a greater speed? § The only difference is mass! § To find mass, use the periodic table

KINETIC ENERGY AND TEMPERATURE § Speed of Gases § Example 1: If CH 4 and NH 3 are both at 284 K, which gas has a greater speed? § Step One: Add up the mass of each gas using the periodic table. § Step Two: The lighter gas moves faster (think about a race between a 100 -pound man and a 700 -pound man, the lighter man would move faster) § Example 2: Which gas has a faster speed between Br 2 and CO 2 if both are at 32°F?
 The kinetic theory of matter states that
The kinetic theory of matter states that Phân độ lown
Phân độ lown Block xoang nhĩ ecg
Block xoang nhĩ ecg Thể thơ truyền thống
Thể thơ truyền thống Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Chiến lược kinh doanh quốc tế của walmart
Chiến lược kinh doanh quốc tế của walmart Tìm độ lớn thật của tam giác abc
Tìm độ lớn thật của tam giác abc Con hãy đưa tay khi thấy người vấp ngã
Con hãy đưa tay khi thấy người vấp ngã Tôn thất thuyết là ai
Tôn thất thuyết là ai Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt 11 free states
11 free states Map of northern united states
Map of northern united states Tyranny
Tyranny Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay