Section 10 5 The Kinetic Molecular Theory The









- Slides: 17

Section 10. 5 The Kinetic Molecular Theory

The Kinetic Molecular Theory In this section… a. Gases and Gas Laws on the Molecular Scale b. Molecular speed, Mass and Temperature c. Gas Effusion and Diffusion d. Nonideal Gases

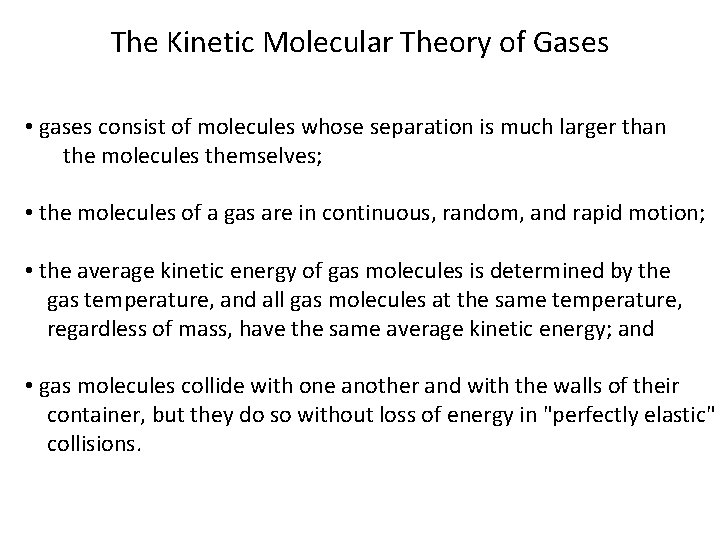
The Kinetic Molecular Theory of Gases • gases consist of molecules whose separation is much larger than the molecules themselves; • the molecules of a gas are in continuous, random, and rapid motion; • the average kinetic energy of gas molecules is determined by the gas temperature, and all gas molecules at the same temperature, regardless of mass, have the same average kinetic energy; and • gas molecules collide with one another and with the walls of their container, but they do so without loss of energy in "perfectly elastic" collisions.

The Kinetic Molecular Theory and Gas Laws Pressure arises from molecule-wall collisions. • P n • P T • P 1/V P n. T/V P = n. RT/V PV = n. RT

The Speed of Gas Molecules: a Derivation Kinetic energy of a particle in motion: Average kinetic energy of a collection of 1 mole of molecules: This equals: R is the gas constant, but with different units R = 8. 314 J/K mol So: And: Therefore: M = molar mass in kg/mol “rms” = root mean square

The Speed of Gas Molecules: a Calculation What is the root mean square speed of O 2 molecules at 20 o. C?

Boltzmann Distributions Do all gas molecules move the same speed? Explore trends.

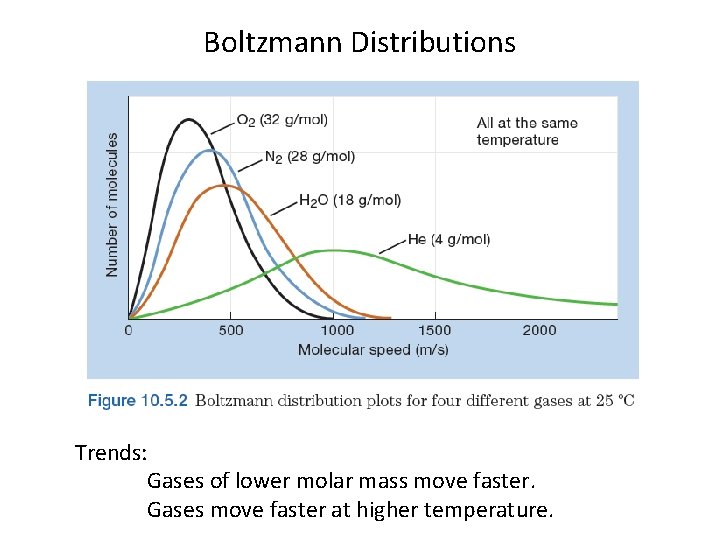
Boltzmann Distributions KEY: Gas molecules move at a range of speeds.

Boltzmann Distributions Trends: Gases of lower molar mass move faster. Gases move faster at higher temperature.

Gas Diffusion Trends: Gases of lower molar mass diffuse faster. Gases diffuse faster at higher temperature.

Gas Diffusion If gas molecules move 100 s of meters per second, why do “smells” spread much more slowly?

Gas Diffusion If gas molecules move 100 s of meters per second, why do “smells” spread much more slowly?

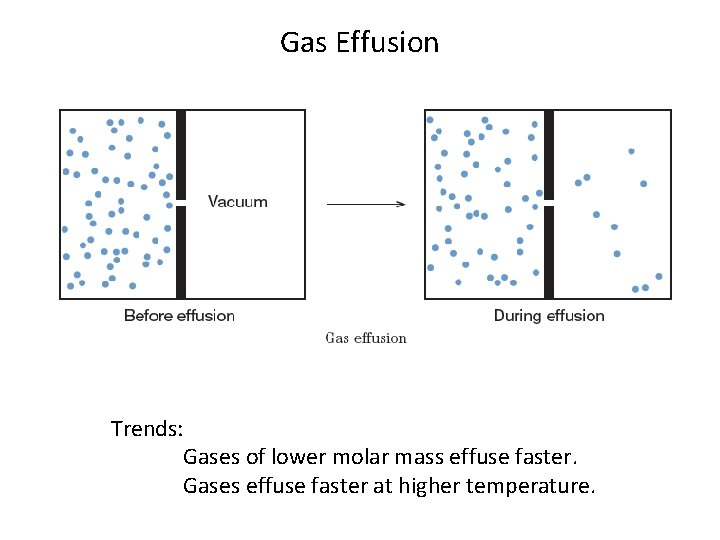
Gas Effusion Trends: Gases of lower molar mass effuse faster. Gases effuse faster at higher temperature.

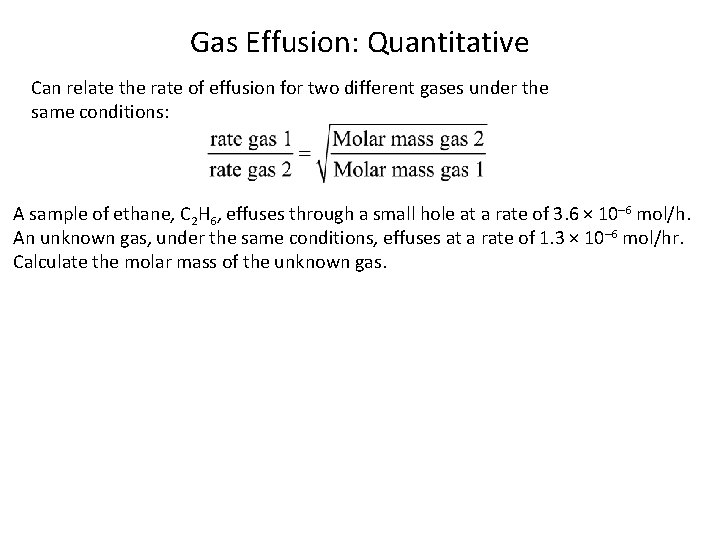
Gas Effusion: Quantitative Can relate the rate of effusion for two different gases under the same conditions: A sample of ethane, C 2 H 6, effuses through a small hole at a rate of 3. 6 × 10– 6 mol/h. An unknown gas, under the same conditions, effuses at a rate of 1. 3 × 10– 6 mol/hr. Calculate the molar mass of the unknown gas.

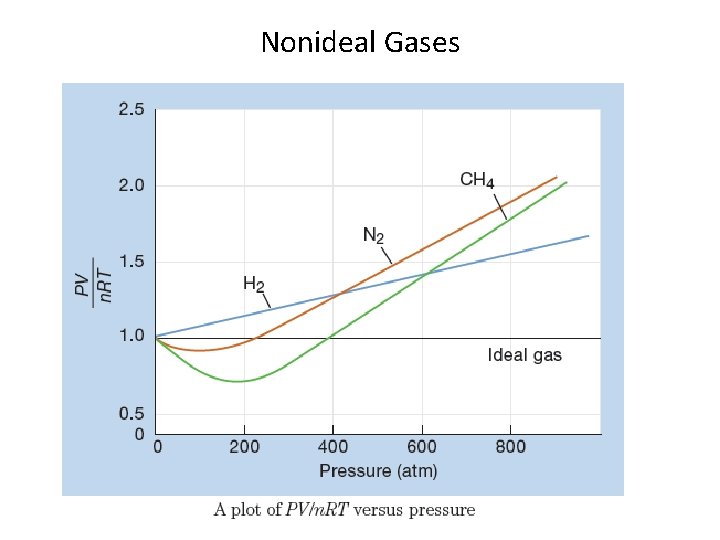
Nonideal Gases Two assumptions of the kinetic molecular theory are sometimes not good: 1. It is assumed that the gas molecules don’t take up any volume and that all the volume is available to all the molecules. This is not true when the gas is at high concentration, which happens when pressure is very high. When this happens, the observed pressure is greater than expected from the ideal gas law. 2. It is assumed that the gas molecules do not interact, that they collide without “sticking. ” This is not true for highly polar molecules and at low temperatures. When this happens, the observed pressure is less than expected from the ideal gas law.

Nonideal Gases

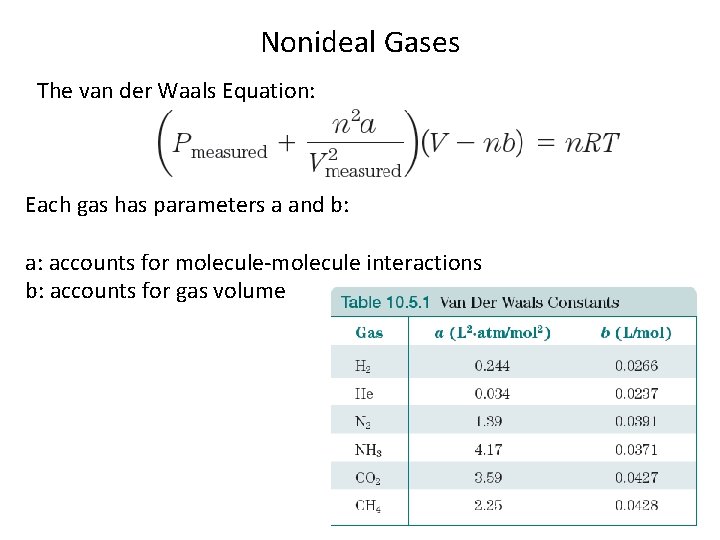
Nonideal Gases The van der Waals Equation: Each gas has parameters a and b: a: accounts for molecule-molecule interactions b: accounts for gas volume