Theories Laws and Hypotheses Gas Laws and Kinetic







- Slides: 21

Theories, Laws, and Hypotheses: Gas Laws and Kinetic Molecular Theory

Define the Terms and Draw the Relationship n Theory n Law n Hypothesis

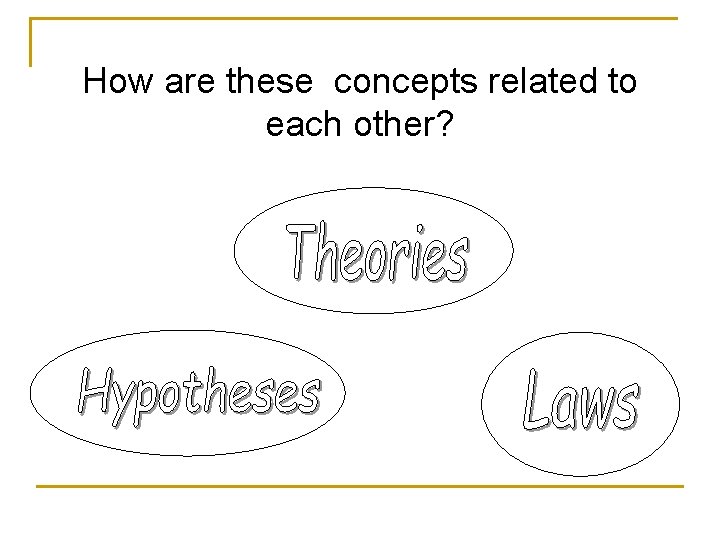
How are these concepts related to each other?

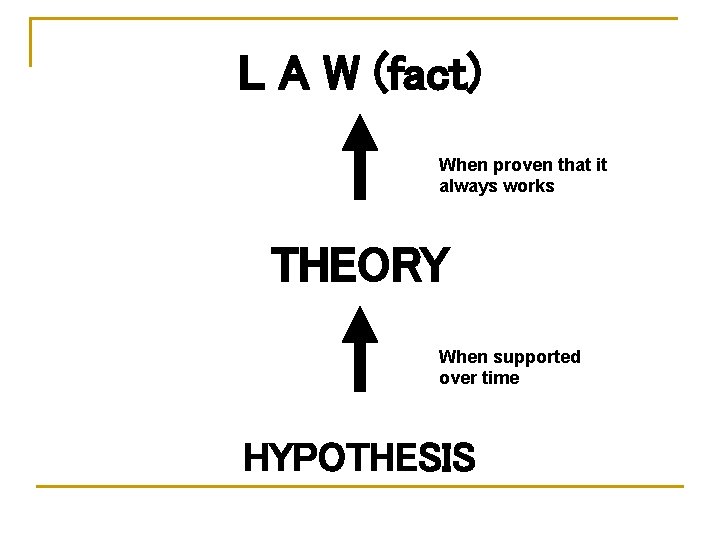
L A W (fact) When proven that it always works THEORY When supported over time HYPOTHESIS

List examples of scientific theories and laws…. Laws Theories

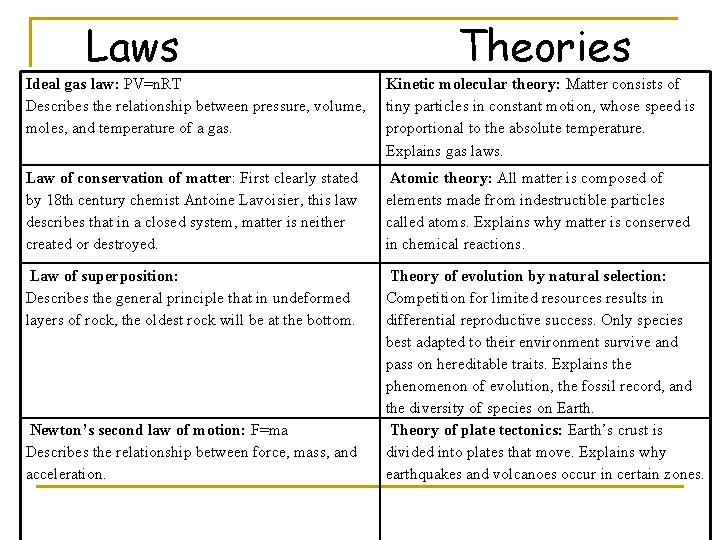
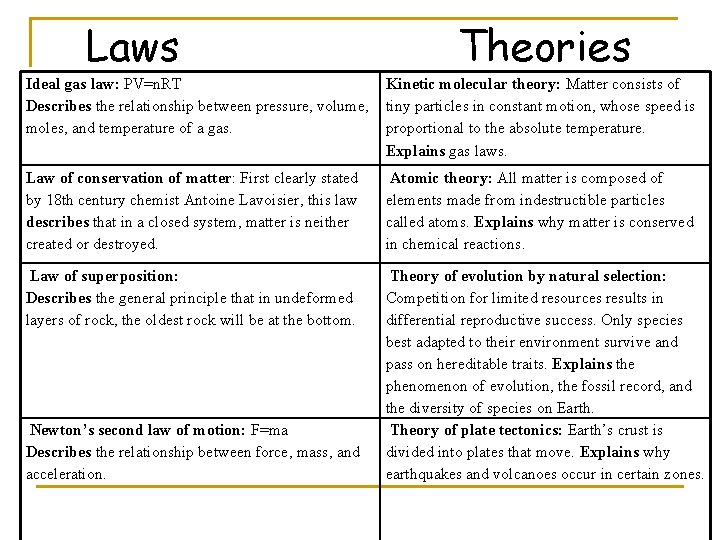
Laws Theories Ideal gas law: PV=n. RT Describes the relationship between pressure, volume, moles, and temperature of a gas. Kinetic molecular theory: Matter consists of tiny particles in constant motion, whose speed is proportional to the absolute temperature. Explains gas laws. Law of conservation of matter: First clearly stated by 18 th century chemist Antoine Lavoisier, this law describes that in a closed system, matter is neither created or destroyed. Atomic theory: All matter is composed of elements made from indestructible particles called atoms. Explains why matter is conserved in chemical reactions. Law of superposition: Describes the general principle that in undeformed layers of rock, the oldest rock will be at the bottom. Theory of evolution by natural selection: Competition for limited resources results in differential reproductive success. Only species best adapted to their environment survive and pass on hereditable traits. Explains the phenomenon of evolution, the fossil record, and the diversity of species on Earth. Theory of plate tectonics: Earth’s crust is divided into plates that move. Explains why earthquakes and volcanoes occur in certain zones. Newton’s second law of motion: F=ma Describes the relationship between force, mass, and acceleration.

Laws Theories Ideal gas law: PV=n. RT Describes the relationship between pressure, volume, moles, and temperature of a gas. Kinetic molecular theory: Matter consists of tiny particles in constant motion, whose speed is proportional to the absolute temperature. Explains gas laws. Law of conservation of matter: First clearly stated by 18 th century chemist Antoine Lavoisier, this law describes that in a closed system, matter is neither created or destroyed. Atomic theory: All matter is composed of elements made from indestructible particles called atoms. Explains why matter is conserved in chemical reactions. Law of superposition: Describes the general principle that in undeformed layers of rock, the oldest rock will be at the bottom. Theory of evolution by natural selection: Competition for limited resources results in differential reproductive success. Only species best adapted to their environment survive and pass on hereditable traits. Explains the phenomenon of evolution, the fossil record, and the diversity of species on Earth. Theory of plate tectonics: Earth’s crust is divided into plates that move. Explains why earthquakes and volcanoes occur in certain zones. Newton’s second law of motion: F=ma Describes the relationship between force, mass, and acceleration.

Brainstorm n Jot down everything you know about the behavior and properties of gases and the gas laws.

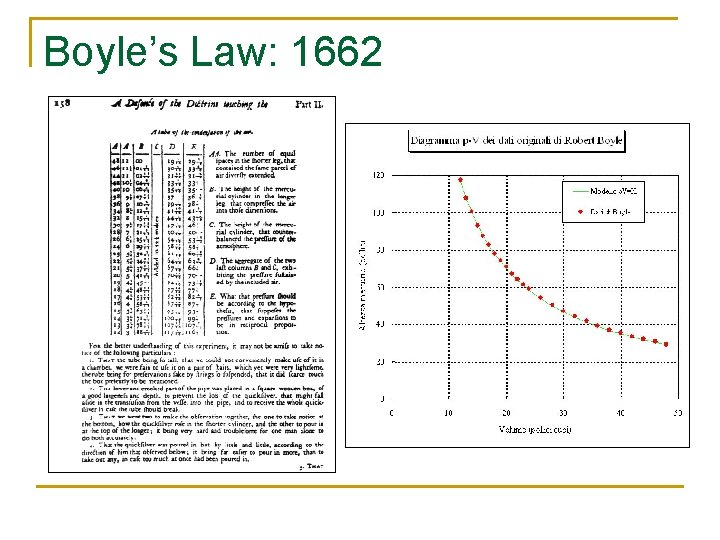
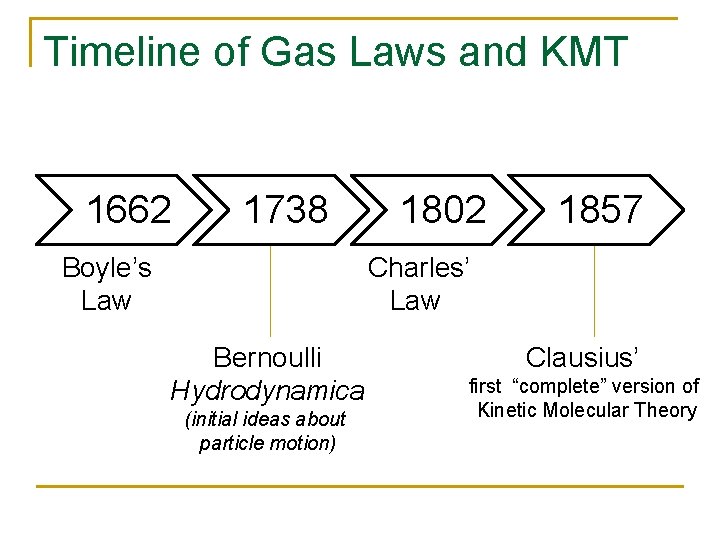
Boyle’s Law 7 investigations deal with changes in pressure as a result of changes in volume New Experiments Physico. Mechanicall, Touching the Spring of the Air, and its Effects. . . (1662)

Boyle’s Law: 1662

Charles’ Law n The Expansion of Gases by Heat. In Annales de Chimie 43, 137 (1802) q First published by Gay. Lussac who attributed it to unpublished work of Charles’ in 1780 s. Image retrieved from: http: //www. freshney. org/ptonline/data/biography/jlgl. htm

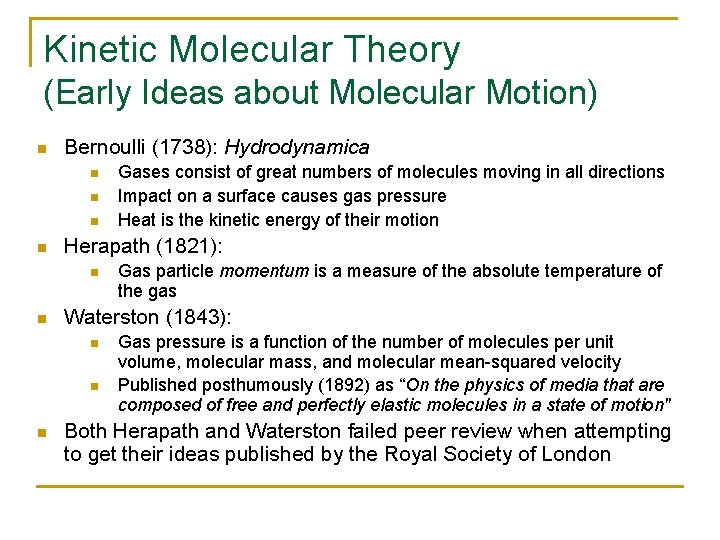
Kinetic Molecular Theory (Early Ideas about Molecular Motion) n Bernoulli (1738): Hydrodynamica n n Herapath (1821): n n Gas particle momentum is a measure of the absolute temperature of the gas Waterston (1843): n n n Gases consist of great numbers of molecules moving in all directions Impact on a surface causes gas pressure Heat is the kinetic energy of their motion Gas pressure is a function of the number of molecules per unit volume, molecular mass, and molecular mean-squared velocity Published posthumously (1892) as “On the physics of media that are composed of free and perfectly elastic molecules in a state of motion" Both Herapath and Waterston failed peer review when attempting to get their ideas published by the Royal Society of London

Kinetic Molecular Theory n Krönig (1850) n n n Included only translational particle motion Gundzüge einer Theorie der Gase Ann. Phys. 79, 368, 500 Clausius (1857) n n n q Included translational, rotational, and vibrational particle motion The size of a particle is negligibly small relative to its container Changes in particle motion due to collisions are infinitesimal relative to time between successive collisions. The influence of the molecular forces must be infinitesimal. Heat is the average kinetic energy of molecules. Published: "Über die bewegende Kraft der Wärme" ("On the Moving Force of Heat and the Laws of Heat which may be Deduced Therefrom”, Annalen der Physik 100, 353 -380

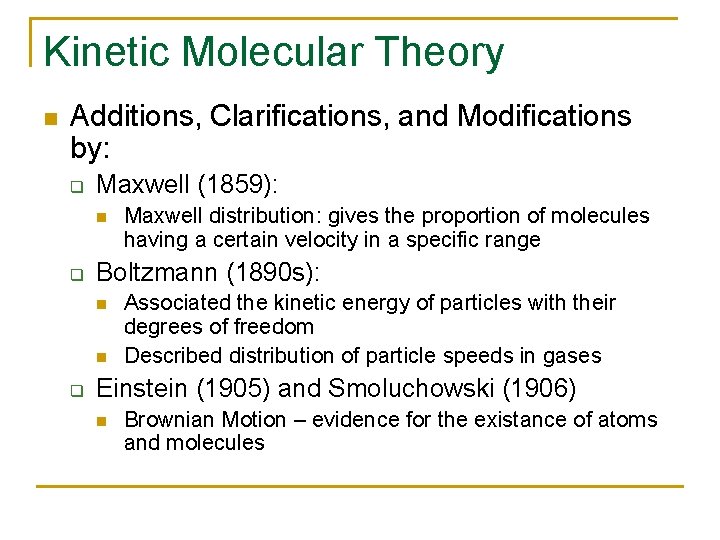
Kinetic Molecular Theory n Additions, Clarifications, and Modifications by: q Maxwell (1859): n q Boltzmann (1890 s): n n q Maxwell distribution: gives the proportion of molecules having a certain velocity in a specific range Associated the kinetic energy of particles with their degrees of freedom Described distribution of particle speeds in gases Einstein (1905) and Smoluchowski (1906) n Brownian Motion – evidence for the existance of atoms and molecules

Timeline of Gas Laws and KMT 1662 1738 Boyle’s Law 1802 1857 Charles’ Law Bernoulli Hydrodynamica (initial ideas about particle motion) Clausius’ first “complete” version of Kinetic Molecular Theory

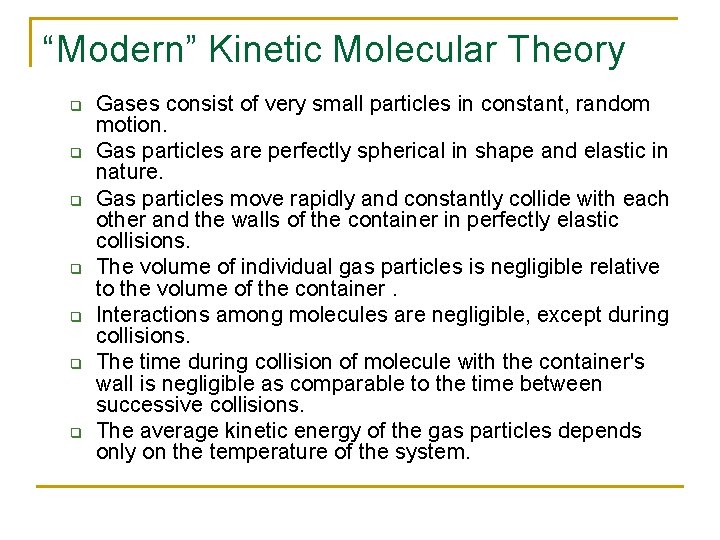
“Modern” Kinetic Molecular Theory q q q q Gases consist of very small particles in constant, random motion. Gas particles are perfectly spherical in shape and elastic in nature. Gas particles move rapidly and constantly collide with each other and the walls of the container in perfectly elastic collisions. The volume of individual gas particles is negligible relative to the volume of the container. Interactions among molecules are negligible, except during collisions. The time during collision of molecule with the container's wall is negligible as comparable to the time between successive collisions. The average kinetic energy of the gas particles depends only on the temperature of the system.

Scientific Hypothesis: 1. A proposed answer to a research question 2. A tentative explanation for an observation or phenomena that can be tested through experimentation.

Scientific Theory: A general principle supported by a substantial body of evidence offered to provide an explanation of observed facts and as a basis for future discussion or investigation. Lincoln, Boxshall, and Clark (1990) Scientific Law: A scientific law is a description of a natural relationship or principle, often expressed in mathematical terms.

L A W (fact) When proven that it always works THEORY When supported over time HYPOTHESIS

THEORY HYPOTHESIS LAW

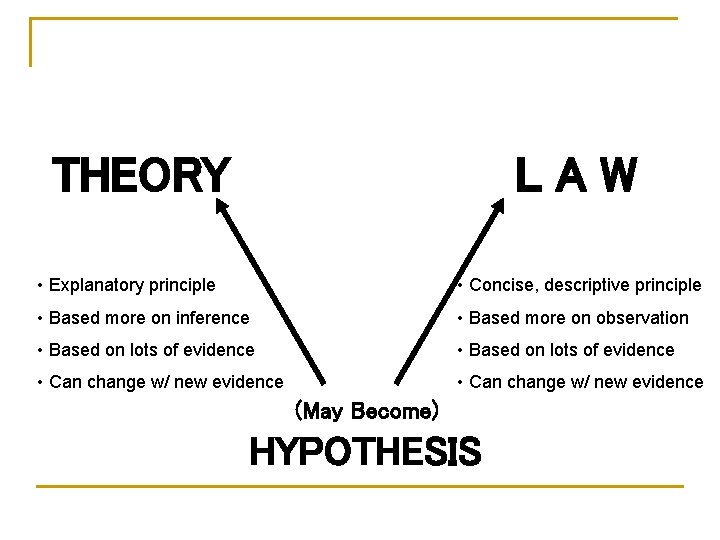
THEORY LAW • Explanatory principle • Concise, descriptive principle • Based more on inference • Based more on observation • Based on lots of evidence • Can change w/ new evidence (May Become) HYPOTHESIS