THE KINETIC THEORY OF MATTER The Kinetic theory




- Slides: 16

THE KINETIC THEORY OF MATTER

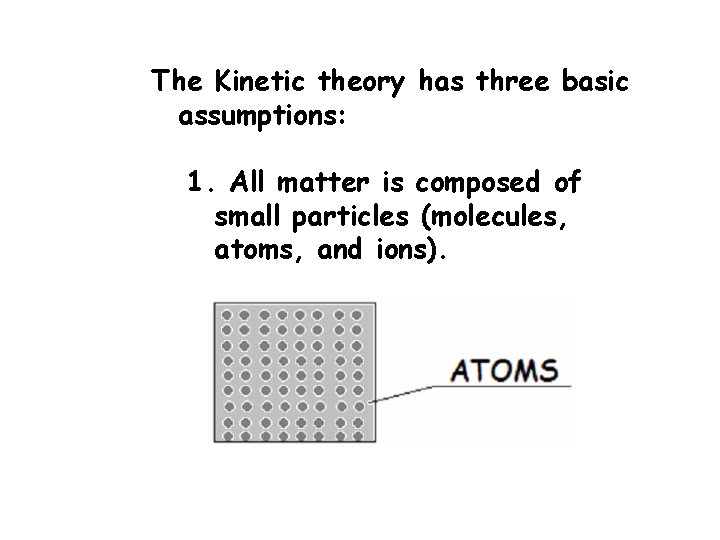
The Kinetic theory has three basic assumptions: 1. All matter is composed of small particles (molecules, atoms, and ions).

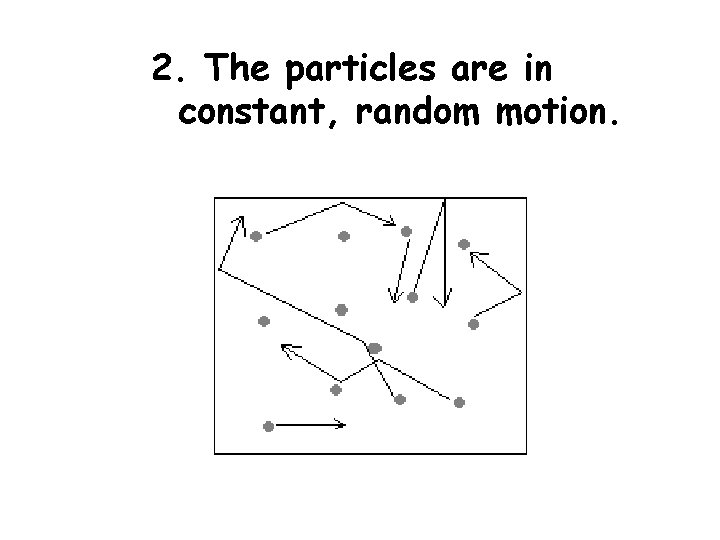
2. The particles are in constant, random motion.

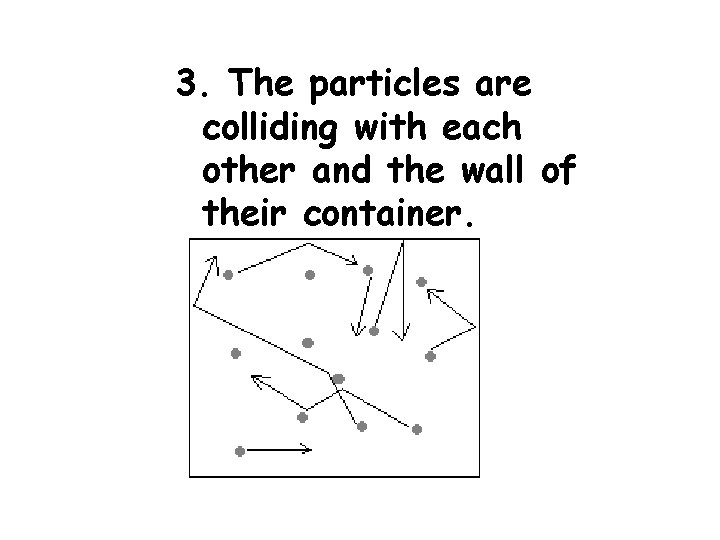
3. The particles are colliding with each other and the wall of their container.

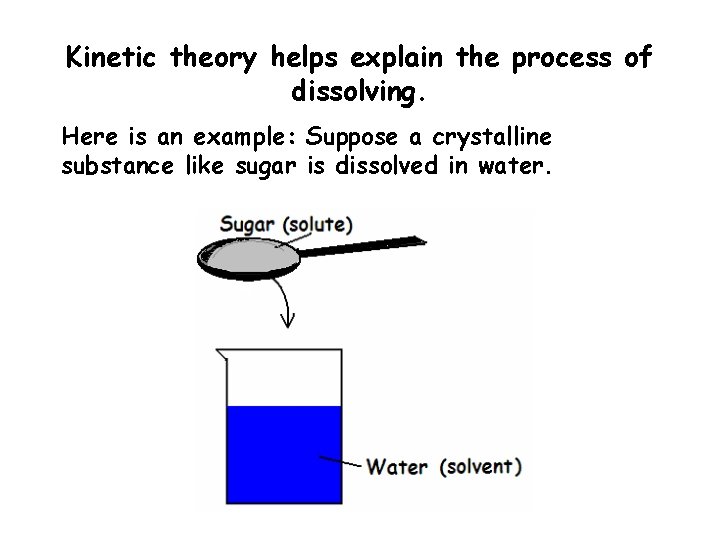
Kinetic theory helps explain the process of dissolving. Here is an example: Suppose a crystalline substance like sugar is dissolved in water.

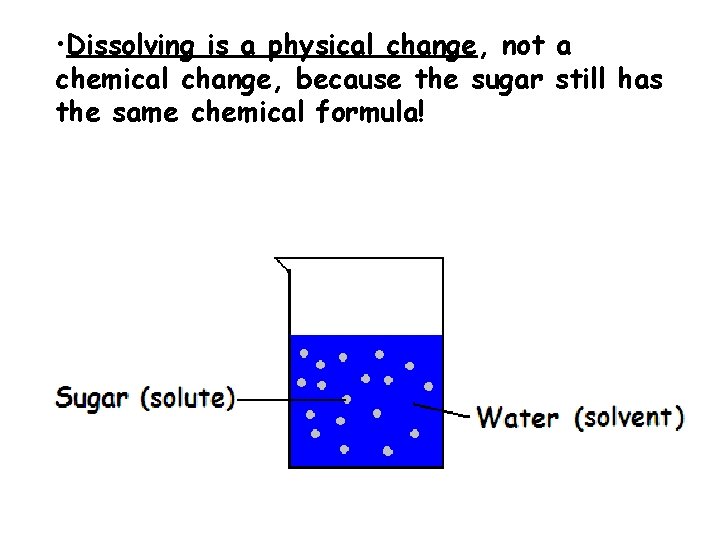
• Dissolving is a physical change, not a chemical change, because the sugar still has the same chemical formula!

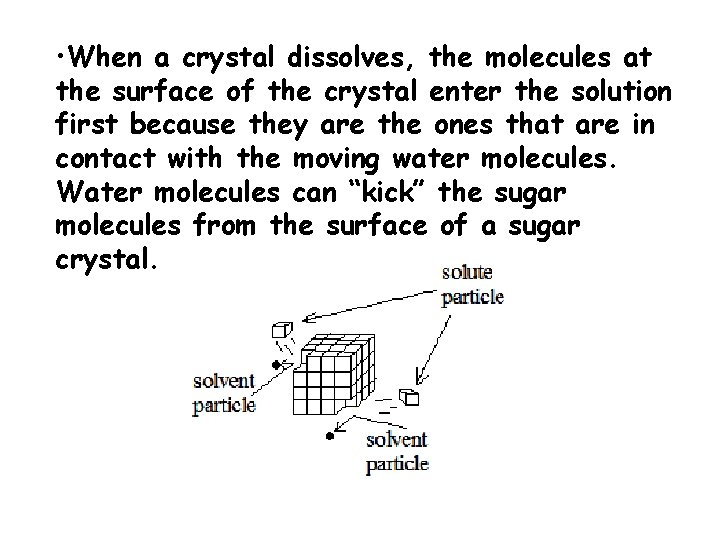
• When a crystal dissolves, the molecules at the surface of the crystal enter the solution first because they are the ones that are in contact with the moving water molecules. Water molecules can “kick” the sugar molecules from the surface of a sugar crystal.

Though there are several ways to increase the rate at which a solute dissolves in a solvent, they all involve increasing the number of particle collisions.

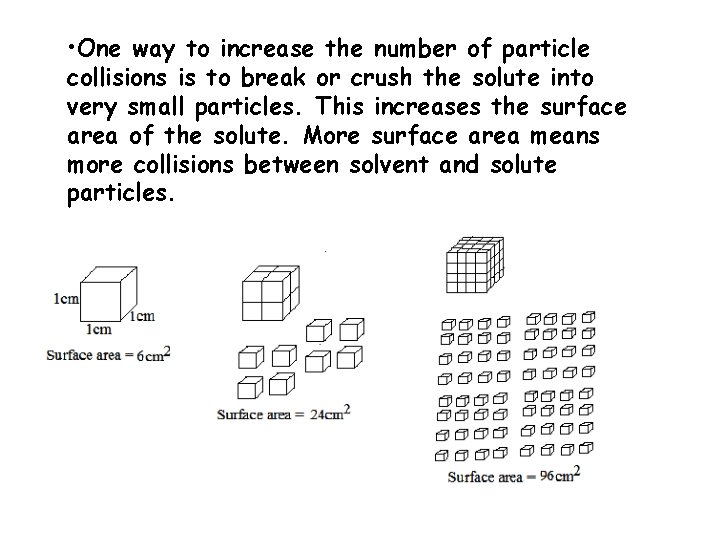
• One way to increase the number of particle collisions is to break or crush the solute into very small particles. This increases the surface area of the solute. More surface area means more collisions between solvent and solute particles.

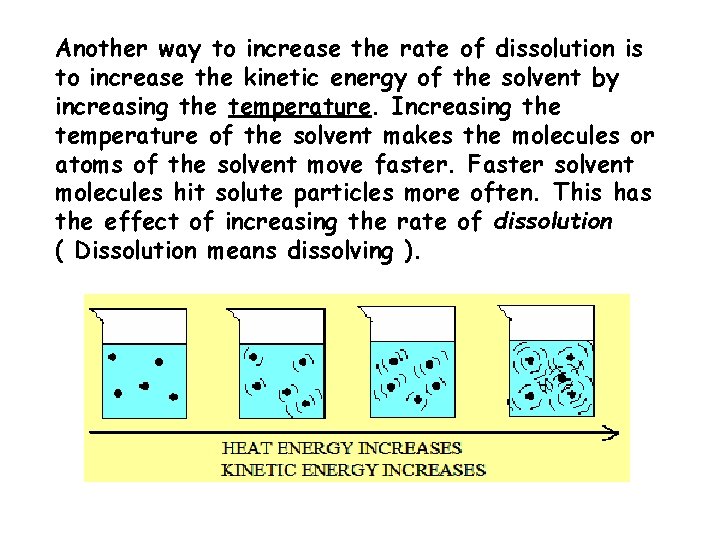
Another way to increase the rate of dissolution is to increase the kinetic energy of the solvent by increasing the temperature. Increasing the temperature of the solvent makes the molecules or atoms of the solvent move faster. Faster solvent molecules hit solute particles more often. This has the effect of increasing the rate of dissolution ( Dissolution means dissolving ).

SUMMARY: If you want to dissolve something faster: A. Add more kinetic energy by warming up the solvent. This increases the number of particle collisions and speeds up dissolving. B. Crush the solute into smaller particles. This increases the number of particle collisions by increase surface area and this speeds up dissolving C. Add more kinetic energy by agitating the solution. This increases the number of particle collisions.

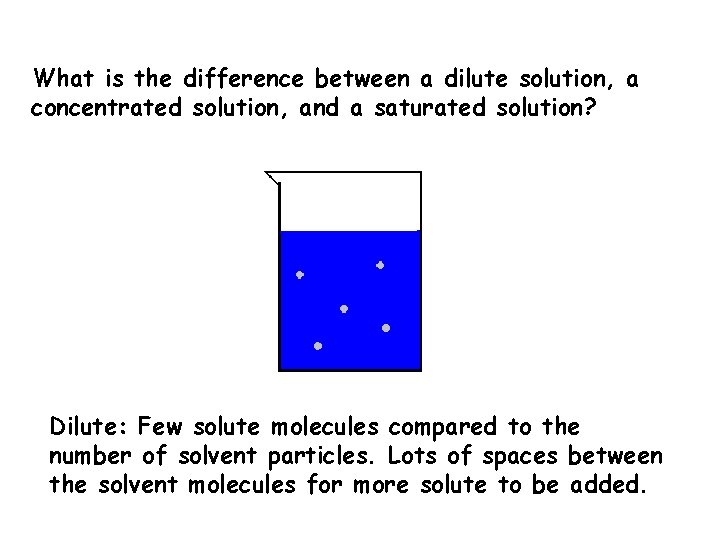
What is the difference between a dilute solution, a concentrated solution, and a saturated solution? Dilute: Few solute molecules compared to the number of solvent particles. Lots of spaces between the solvent molecules for more solute to be added.

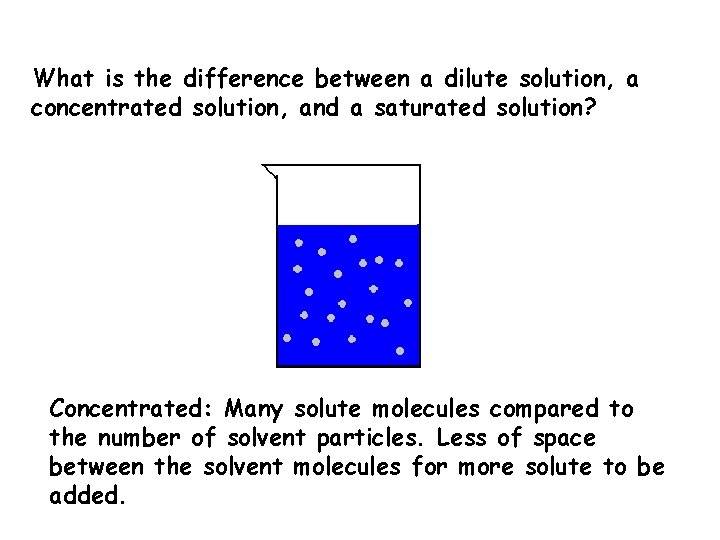
What is the difference between a dilute solution, a concentrated solution, and a saturated solution? Concentrated: Many solute molecules compared to the number of solvent particles. Less of space between the solvent molecules for more solute to be added.

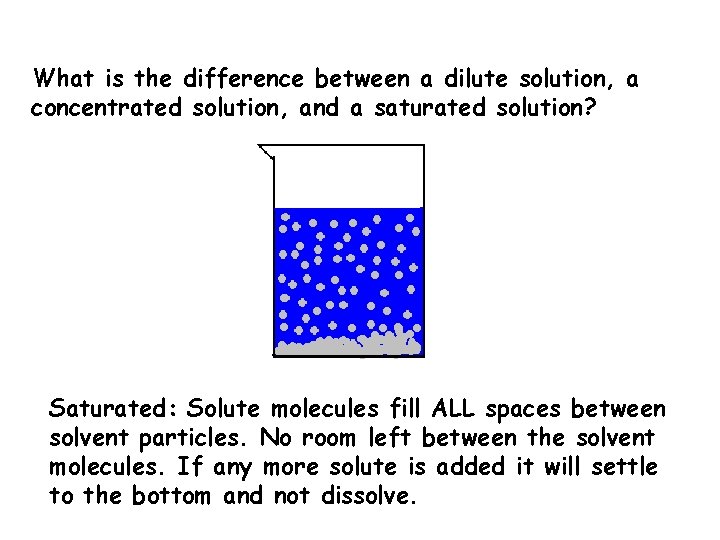
What is the difference between a dilute solution, a concentrated solution, and a saturated solution? Saturated: Solute molecules fill ALL spaces between solvent particles. No room left between the solvent molecules. If any more solute is added it will settle to the bottom and not dissolve.

What's the Difference between "Dissolving" and "Melting"? When a solid dissolves into a solvent, it does not melt since no heat has to be added. It does not change phase, but simply gets broken apart into invisible molecules or atoms that are mixed into the solvent. In order to melt a solid, heat must be added to raise its temperature above its melting point. No other substance is involved.

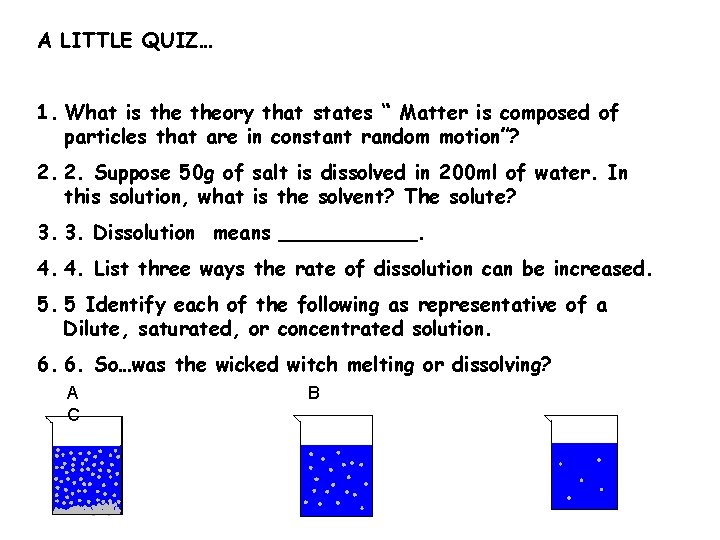
A LITTLE QUIZ… 1. What is theory that states “ Matter is composed of particles that are in constant random motion”? 2. 2. Suppose 50 g of salt is dissolved in 200 ml of water. In this solution, what is the solvent? The solute? 3. 3. Dissolution means ______. 4. 4. List three ways the rate of dissolution can be increased. 5. 5 Identify each of the following as representative of a Dilute, saturated, or concentrated solution. 6. 6. So…was the wicked witch melting or dissolving? A C B
 The kinetic theory of matter states that
The kinetic theory of matter states that Kinetic theory of matter definition
Kinetic theory of matter definition Define kinetic theory of matter
Define kinetic theory of matter Buoyancyability
Buoyancyability Particle theory freezing
Particle theory freezing What is a kinetic theory of matter
What is a kinetic theory of matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Grey matter
Grey matter Composition of matter section 1
Composition of matter section 1 Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers Arbor vitae
Arbor vitae Section 1 composition of matter
Section 1 composition of matter Gray matter and white matter
Gray matter and white matter Grey matter and white matter in brain
Grey matter and white matter in brain Energy naturally flows from warmer matter to cooler matter
Energy naturally flows from warmer matter to cooler matter Kinetic molecular theory of solid
Kinetic molecular theory of solid Kinetic molecular theory of gases
Kinetic molecular theory of gases