Unit 11 Gases Kinetic Molecular Theory of Gases












- Slides: 23

Unit 11: Gases: Kinetic Molecular Theory of Gases

Kinetic Molecular Theory • a theory based on the idea that particles of matter are always in motion.

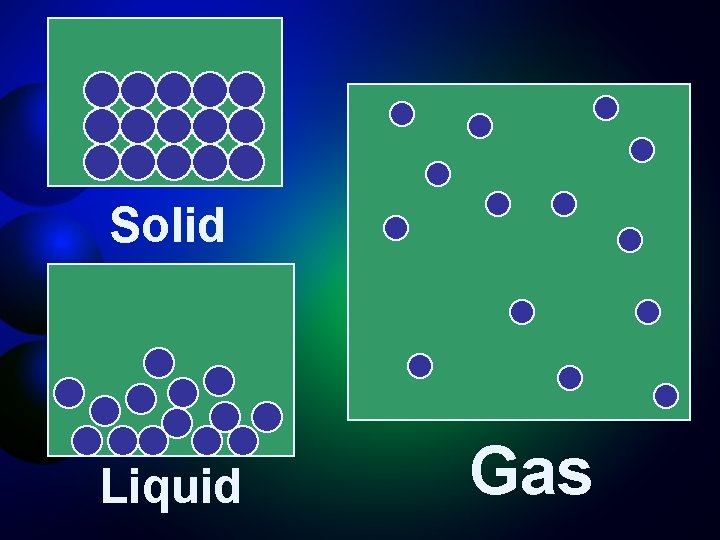
Solid Liquid Gas

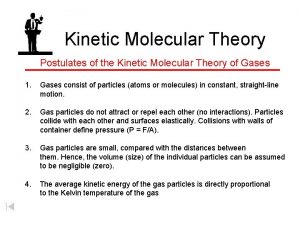
1. Gases consist of a large number of tiny particles that are far apart relative to their size.

2. Collisions between gas particles or gas particles and container walls are elastic collisions.

Elastic Collision: A collision where there is no net loss of kinetic energy.

3. Particles are in constant, rapid, random motion.

4. There are no forces of repulsion or attraction between the particles. (like billiard balls)

5. Kinetic energy (KE) of the particles depends on the temperature. 2 KE = ½ mv

More heat = faster moving particles = higher KE

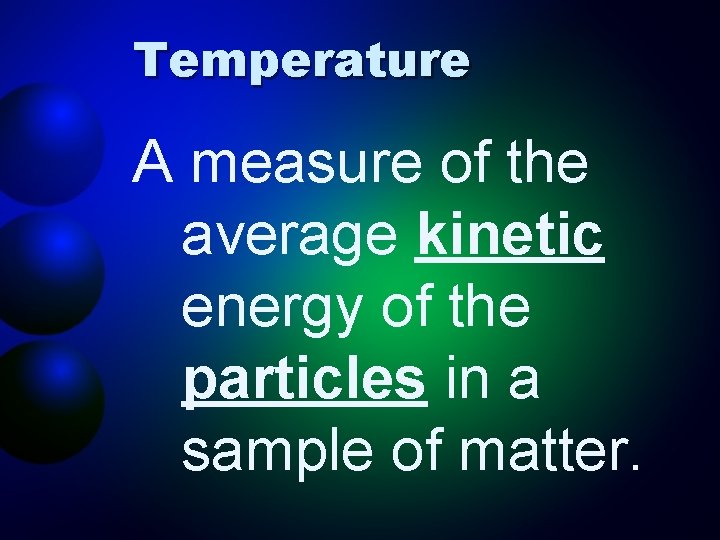
Temperature A measure of the average kinetic energy of the particles in a sample of matter.

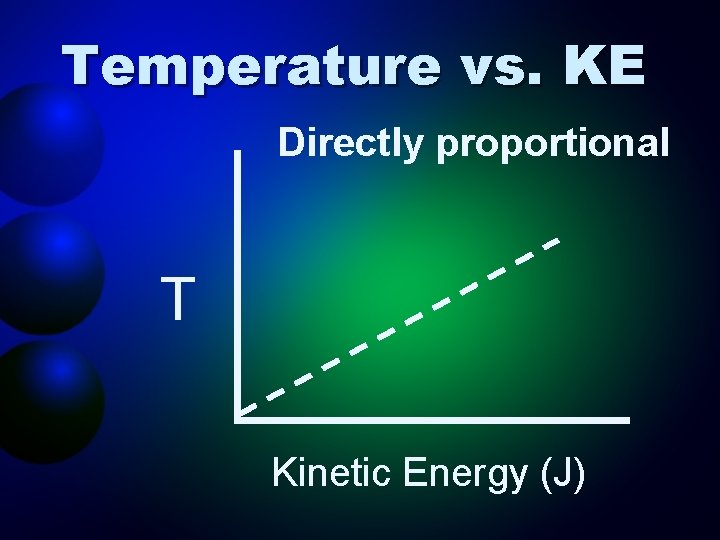
Temperature vs. KE Directly proportional T Kinetic Energy (J)

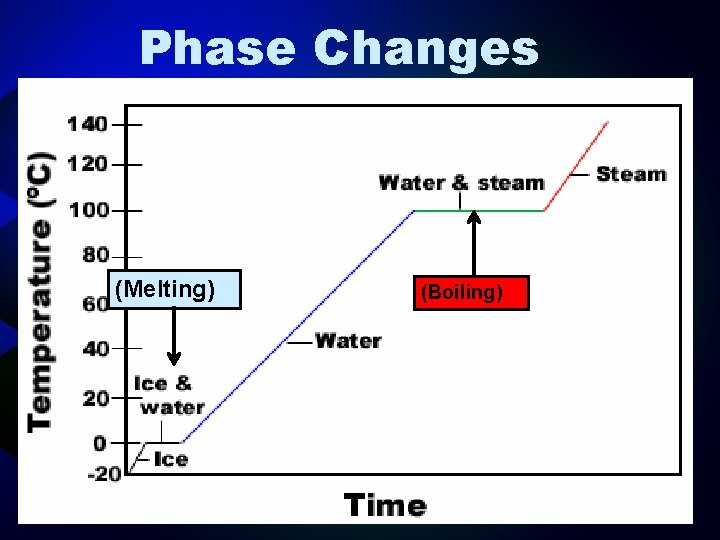
Substances undergo phase changes at characteristic temperatures. The temperature of a pure substance is constant during phase changes.

Phase Changes (Melting) (Boiling)

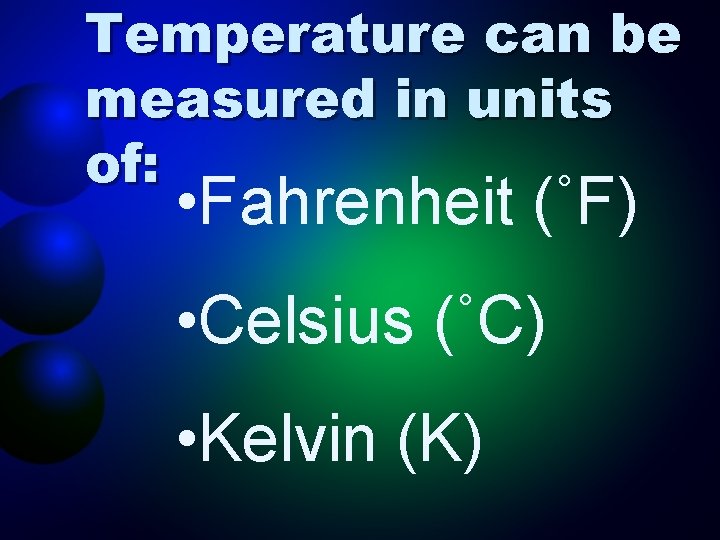
Temperature can be measured in units of: • Fahrenheit (˚F) • Celsius (˚C) • Kelvin (K)

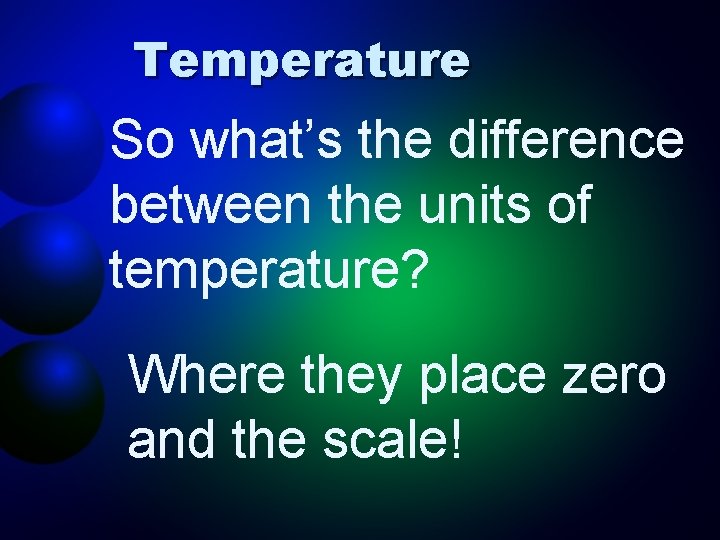
Temperature So what’s the difference between the units of temperature? Where they place zero and the scale!

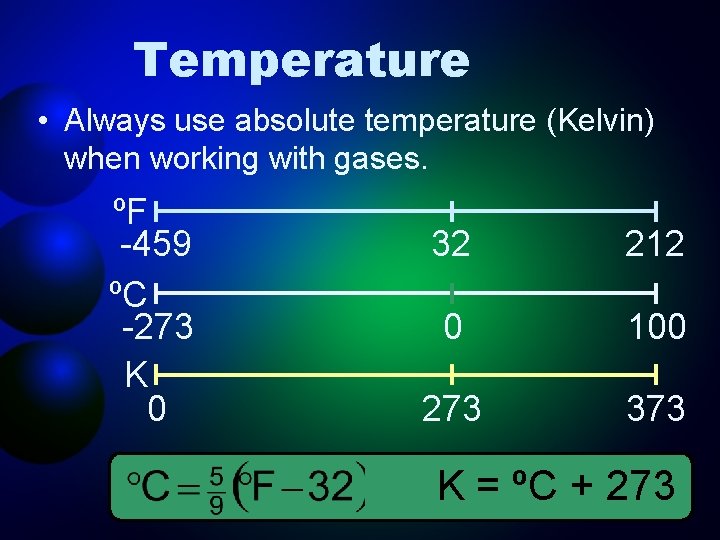
Temperature • Always use absolute temperature (Kelvin) when working with gases. ºF -459 ºC -273 K 0 32 212 0 100 273 373 K = ºC + 273

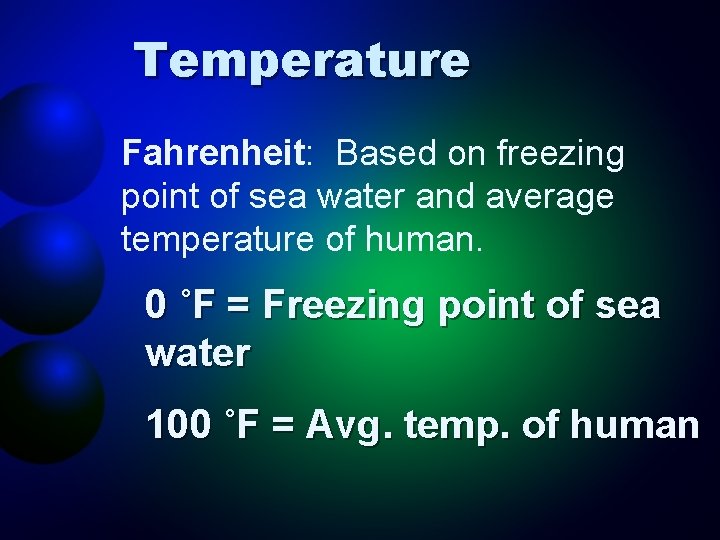
Temperature Fahrenheit: Based on freezing point of sea water and average temperature of human. 0 ˚F = Freezing point of sea water 100 ˚F = Avg. temp. of human

Temperature Celsius: Based on freezing and boiling points of water. 0˚ C = Freezing point of water 100 ˚C = Boiling point of water (at sea level)

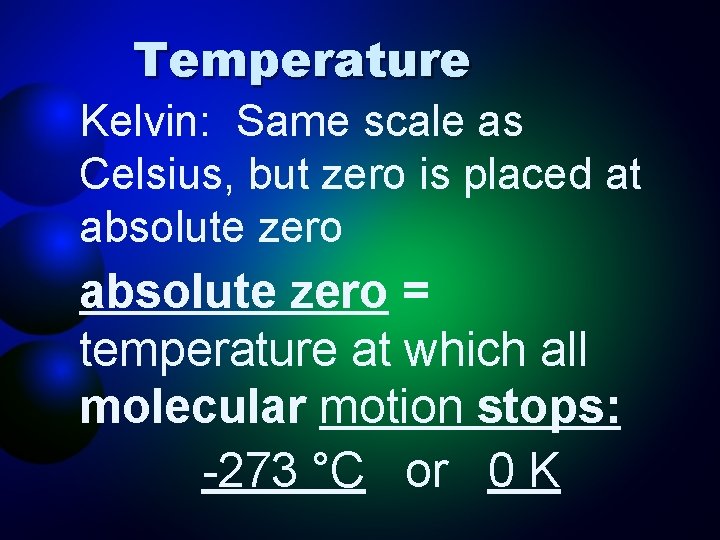
Temperature Kelvin: Same scale as Celsius, but zero is placed at absolute zero = temperature at which all molecular motion stops: -273 °C or 0 K

Calculations for gases use the Kelvin temperature scale in which zero is the coldest temperature possible (absolute zero).

TK = TC + 273 Practice: 25˚C = ? K Ans: 298 K

TK = TC + 273 Practice: 328 K = ? ˚C Ans: 55 ˚C
 Kinetic molecular theory
Kinetic molecular theory Kinetic molecular theory of solid
Kinetic molecular theory of solid Kinetic molecular theory volume
Kinetic molecular theory volume Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Kinetic energy molecular theory
Kinetic energy molecular theory Kinetic theory def
Kinetic theory def Theory vs hypothesis
Theory vs hypothesis Charles law in terms of kinetic molecular theory
Charles law in terms of kinetic molecular theory Basic postulates of kinetic theory of gases
Basic postulates of kinetic theory of gases Kinetic molecular theory
Kinetic molecular theory Write postulates of kinetic theory of gases
Write postulates of kinetic theory of gases Pv=1/3nmc^2
Pv=1/3nmc^2 Kinetic molecular theory
Kinetic molecular theory Tenets of kinetic molecular theory
Tenets of kinetic molecular theory Particle theory melting
Particle theory melting Kinetic theory of gases
Kinetic theory of gases Kinetic theory
Kinetic theory Postulates of kinetic theory of gases
Postulates of kinetic theory of gases General gas equation is
General gas equation is Molecular theory of gases and liquids
Molecular theory of gases and liquids Vbt vs mot theory
Vbt vs mot theory Vb theory vs mo theory
Vb theory vs mo theory Valence bond theory and molecular orbital theory
Valence bond theory and molecular orbital theory