KINETIC THEORY OF GASES KINETIC THEORY OF GASES










- Slides: 38

KINETIC THEORY OF GASES

KINETIC THEORY OF GASES GAS LAWS

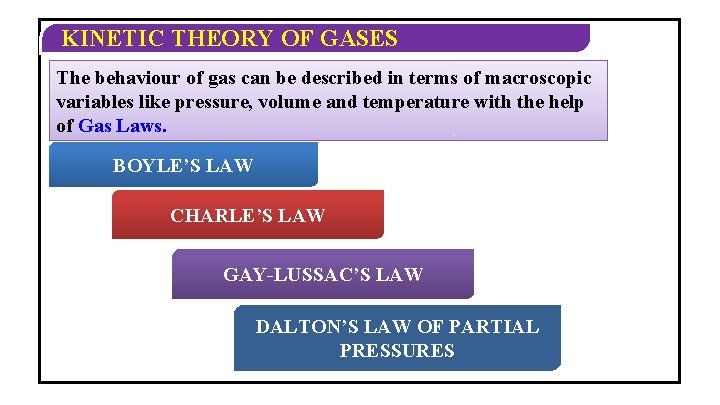
KINETIC THEORY OF GASES The behaviour of gas can be described in terms of macroscopic variables like pressure, volume and temperature with the help of Gas Laws. BOYLE’S LAW CHARLE’S LAW GAY-LUSSAC’S LAW DALTON’S LAW OF PARTIAL PRESSURES

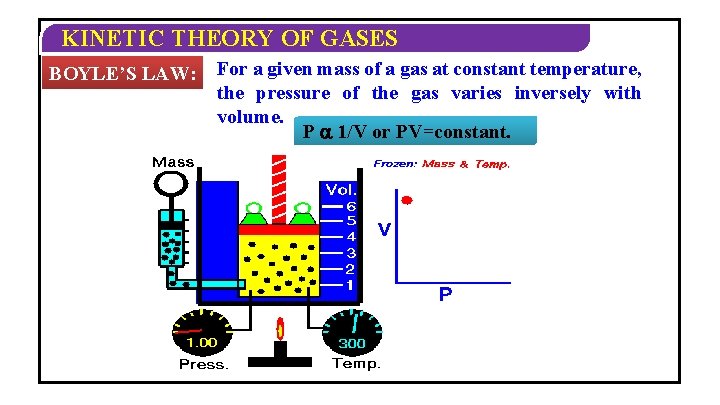
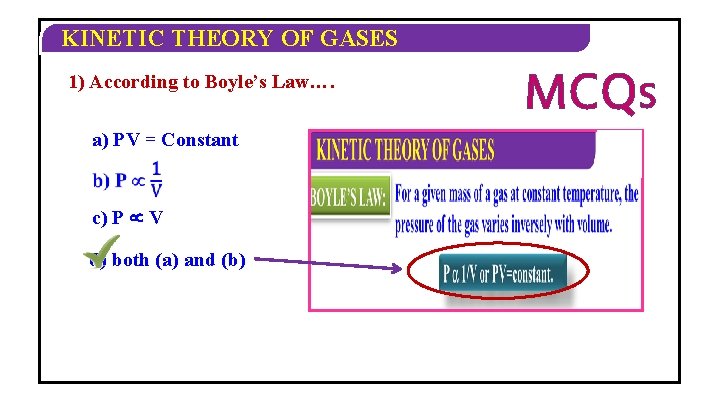
KINETIC THEORY OF GASES BOYLE’S LAW: For a given mass of a gas at constant temperature, the pressure of the gas varies inversely with volume. P 1/V or PV=constant.

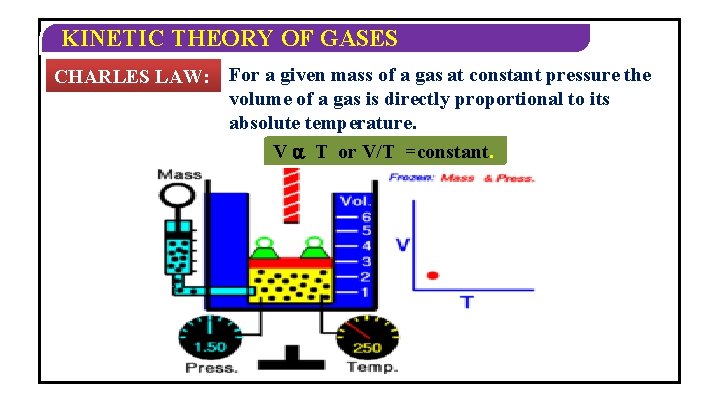
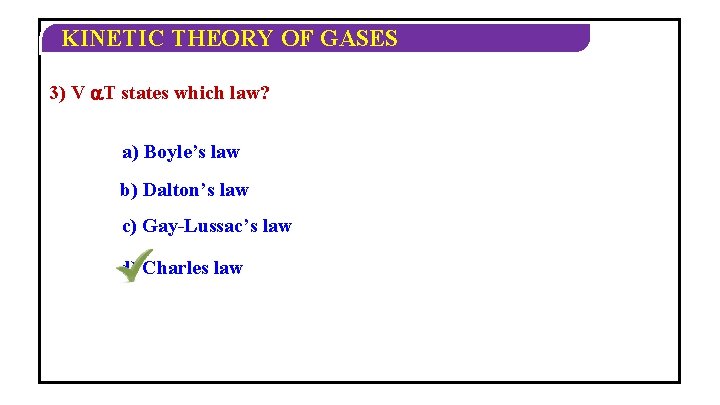
KINETIC THEORY OF GASES CHARLES LAW: For a given mass of a gas at constant pressure the volume of a gas is directly proportional to its absolute temperature. V T or V/T =constant.

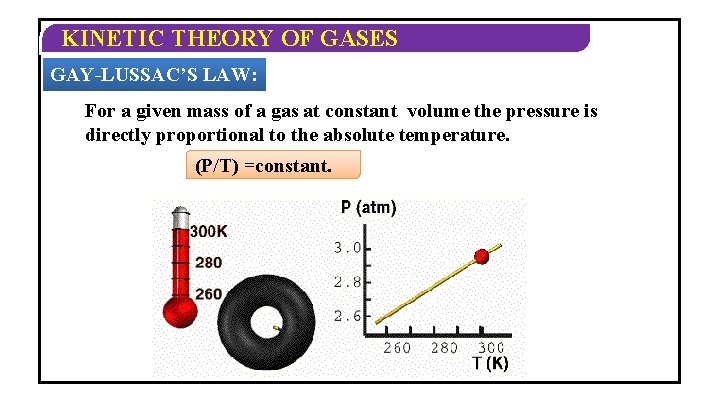
KINETIC THEORY OF GASES GAY-LUSSAC’S LAW: For a given mass of a gas at constant volume the pressure is directly proportional to the absolute temperature. (P/T) =constant.

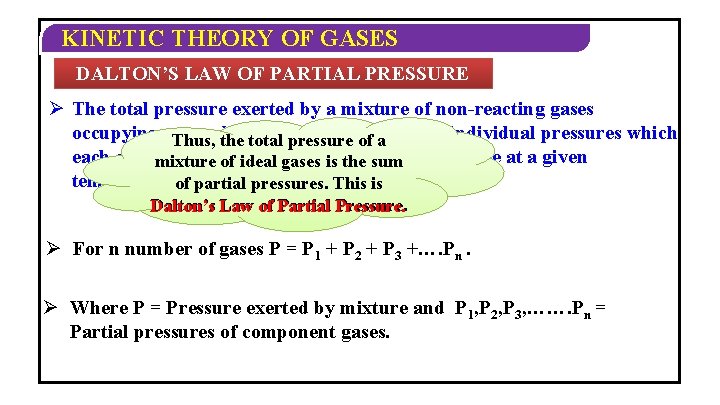
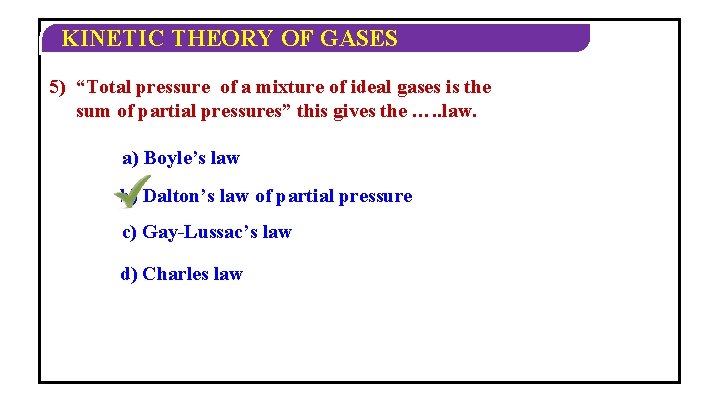
KINETIC THEORY OF GASES DALTON’S LAW OF PARTIAL PRESSURE Ø The total pressure exerted by a mixture of non-reacting gases occupying a. Thus, vesselthe is total equalpressure to the sum of a of the individual pressures which each gas exert if itofalone occupies same volume at a given mixture ideal gases is thethe sum temperature. of partial pressures. This is Dalton’s Law of Partial Pressure. Ø For n number of gases P = P 1 + P 2 + P 3 +…. Pn. Ø Where P = Pressure exerted by mixture and P 1, P 2, P 3, ……. Pn = Partial pressures of component gases.

KINETIC THEORY OF GASES 1) According to Boyle’s Law…. a) PV = Constant c) P µ V d) both (a) and (b) MCQS

KINETIC THEORY OF GASES 2) Necessary condition for Boyle’s law is …… a) constant temperature b) constant volume c) constant pressure d) none of these

KINETIC THEORY OF GASES 3) V T states which law? a) Boyle’s law b) Dalton’s law c) Gay-Lussac’s law d) Charles law

KINETIC THEORY OF GASES 4) P/T = constant gives the…. . law. a) Boyle’s law b) Dalton’s law c) Gay-Lussac’s law d) Charles law

KINETIC THEORY OF GASES 5) “Total pressure of a mixture of ideal gases is the sum of partial pressures” this gives the …. . law. a) Boyle’s law b) Dalton’s law of partial pressure c) Gay-Lussac’s law d) Charles law

KINETIC THEORY OF GASES ASSUMPTIONS OF KINETIC THEORY OF GASES

KINETIC THEORY OF GASES Assumptions of kinetic theory of gas Ø Gas consists of large number of tiny particles known as molecules. .

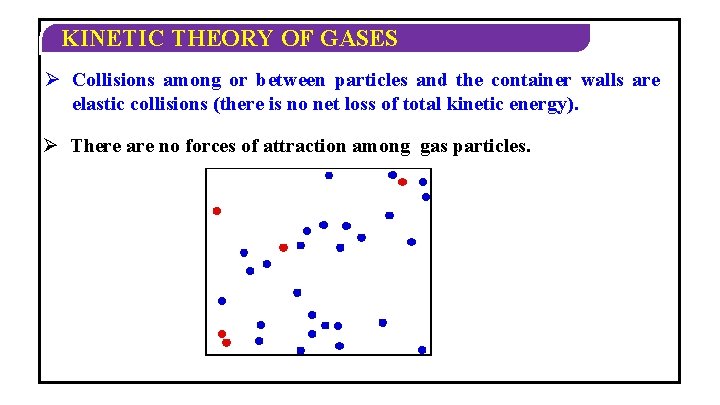
KINETIC THEORY OF GASES Ø Collisions among or between particles and the container walls are elastic collisions (there is no net loss of total kinetic energy). Ø There are no forces of attraction among gas particles.

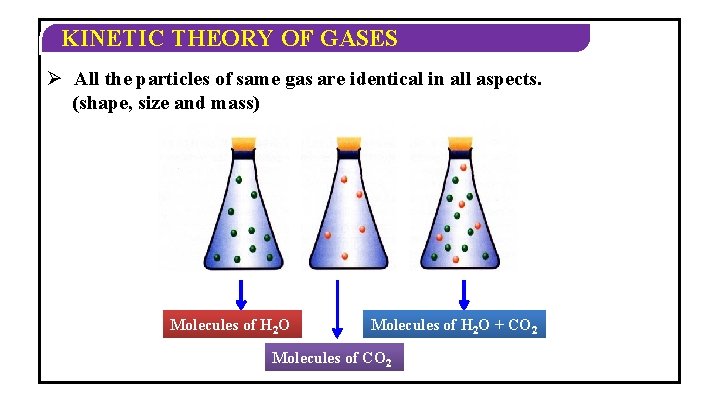
KINETIC THEORY OF GASES Ø All the particles of same gas are identical in all aspects. (shape, size and mass) Molecules of H 2 O + CO 2 Molecules of CO 2

KINETIC THEORY OF GASES Ø Pressure is constant in all the directions.

KINETIC THEORY OF GASES Ø Gas particles are continuous, rapid and are in random motion. Therefore, they possess kinetic energy, which is energy of motion. Ø The temperature of the gas depends on the average kinetic energy in it. Ø Molecules obey Newton’s laws of motion.

KINETIC THEORY OF GASES This implies move that total The molecules freely kinetic lines energy is in straight obeying conserved Newton's first. law. total momentum is Since the. The collisions are considered to be conserved as usual. elastic, we can derive an expression for the pressure of a gas based on these assumptions of kinetic theory.

KINETIC THEORY OF GASES 1) Which of the following is the assumption of kinetic theory of gases? a) Gas consists of molecules b) Collisions among the particles are elastic c) Pressure is constant in all directions d) all the above MCQS

KINETIC THEORY OF GASES 2) The temperature of the gas depends on the……. a) average kinetic energy b) average potential energy c) total energy d) none of these

KINETIC THEORY OF GASES PRESSURE OF AN IDEAL GAS

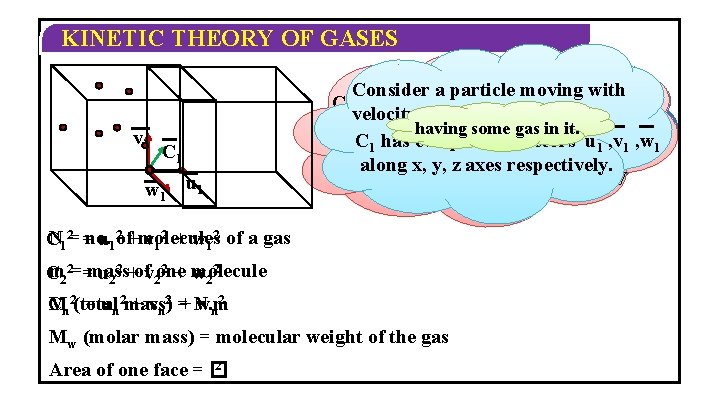
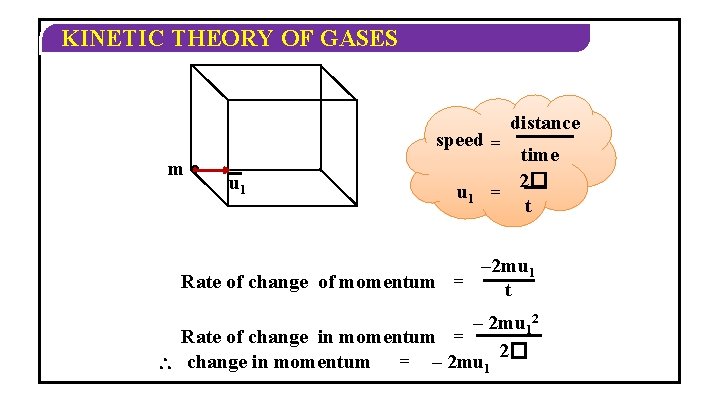
KINETIC THEORY OF GASES v 1 C 1 w 1 u 1 Consider a particle moving with Consider another particle C moving Similarly particle has 3 with Consider velocity C 1. a container n velocity C 2. vectors C 2 has velocity along having some gascomponent in it. x, y, z C 1 has component vectors u , v , w vectors axes un vn wun 2 , v 2 , w 2 1 1 1 along x, x, y, y, z axes respectively. along z axes respectively N 12= =no. C u 12 of+molecules v 12 + w 12 of a gas 2 + molecule m 22==mass C u 22 +ofv 2 one w 22 Mn 2(total C = un 2 mass) + vn 2 =+ N. m wn 2 Mw (molar mass) = molecular weight of the gas 2 Area of one face = �

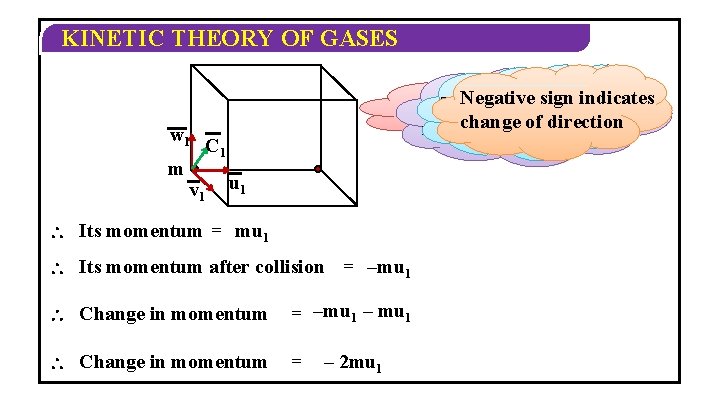
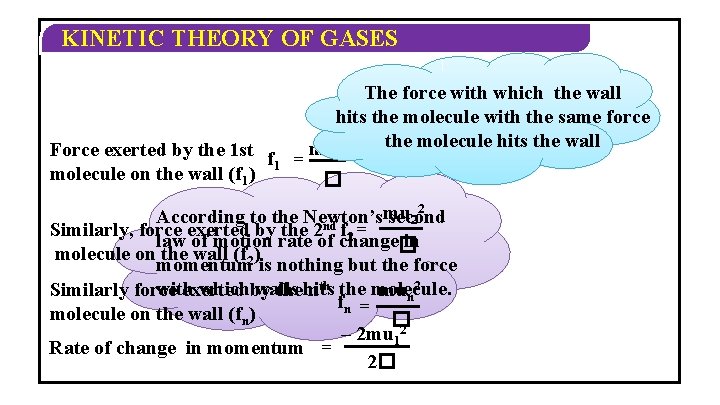
KINETIC THEORY OF GASES w 1 m v 1 Negative indicates m be the mass The particle hits the along wall 1 st. Let molecule issign moving change of direction of the molecule and bounces backu 1 x-axis with velocity C 1 u 1 Its momentum = mu 1 Its momentum after collision = –mu 1 Change in momentum = –mu 1 – mu 1 Change in momentum = – 2 mu 1

KINETIC THEORY OF GASES speed = m u 1 distance time 2� = t – 2 mu 1 Rate of change of momentum = t – 2 mu 12 Rate of change in momentum = 2� change in momentum = – 2 mu 1

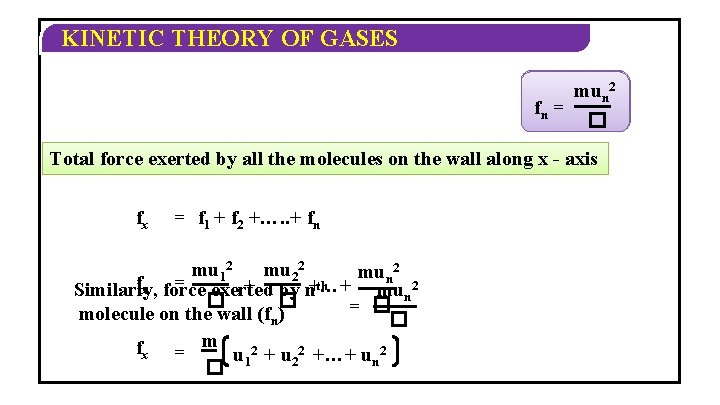
KINETIC THEORY OF GASES The force with which the wall hits the molecule with the same force the molecule hits the wall mu 2 Force exerted by the 1 st f 1 1 = molecule on the wall (f 1) � 2 mu According to the Newton’s second 2 Similarly, force exerted by the 2 nd f 2 = law of motion rate of change� in molecule on the wall (f 2) momentum is nothing but the force with whichby walls Similarly force exerted the hits nth the molecule. mun 2 fn = molecule on the wall (fn) � – 2 mu 12 Rate of change in momentum = 2�

KINETIC THEORY OF GASES mun 122 fn 12 = � Total force exerted by all the molecules on the wall along x - axis fx = f 1 + f 2 +…. . + fn mu 12 mu 22 mun 2 fx force = exerted th + by n+…+ Similarly, mun 2 � � = � molecule on the wall (fn) � fx = m u 2 +…+ u 2 2 n � 1

KINETIC THEORY OF GASES Area of one 2 face of a cube = � Pressure exerted by molecules on the wall along x - axis m u 12 + u 22 +…+ un 2 f � Px = 2 � A (fx) = m u 2 +…+ u 2 2 n � 1

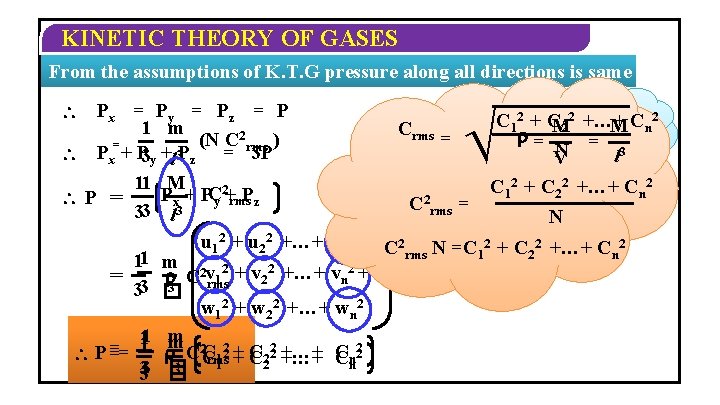
KINETIC THEORY OF GASES Pressure alongx-axis z-axis Pressurealong y-axis m u 12 + u 22 +…+ un 2 m 2 +…+ u 2 fx u 12� + u PPx = 2 n 3 = x = � 2 � A Py = Pz = m 3 � v 12 + v 22 +…+ vn 2 w 12 + w 22 +…+ wn 2

KINETIC THEORY OF GASES From the assumptions of K. T. G pressure along all directions is same Px = Py = Pz = P 1 m (N C 2 rms ) = Px + P 3 y +l 3 Pz = 3 P 11 M Px + PCy 2+rms. Pz P == 33 l 3 11 == 33 1 1 = P == 33 Crms = m. N=M C 2 rms = 2 C 12 + CM 22 +…+ M Cn = = 3 N l V C 12 + C 22 +…+ Cn 2 N u 12 + u 22 +…+ un 2 + C 2 N =C 2 +…+ C 2 rms 1 2 n m 2 2 3 C vrms 1 + v 2 +…+ vn + � w 12 + w 22 +…+ wn 2 m m 2 22 2+ +C C 222 +…+ C 3 C 2 C 1 n Crms +…+ C 1 2 n � 3 �

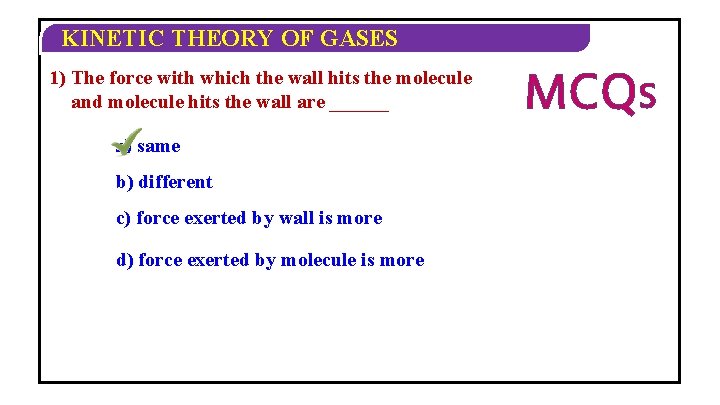
KINETIC THEORY OF GASES 1) The force with which the wall hits the molecule and molecule hits the wall are ______ a) same b) different c) force exerted by wall is more d) force exerted by molecule is more MCQS

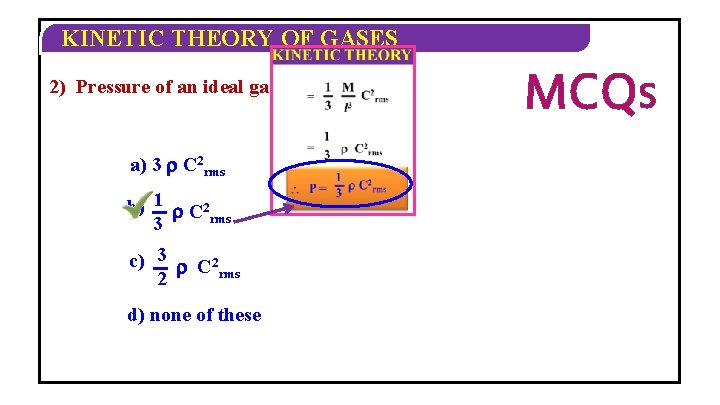
KINETIC THEORY OF GASES 2) Pressure of an ideal gas P = ______ a) 3 C 2 rms b) 1 C 2 rms 3 c) 3 C 2 rms 2 d) none of these MCQS

KINETIC THEORY OF GASES DIFFERENT FORMS OF PRESSURE EQUATION

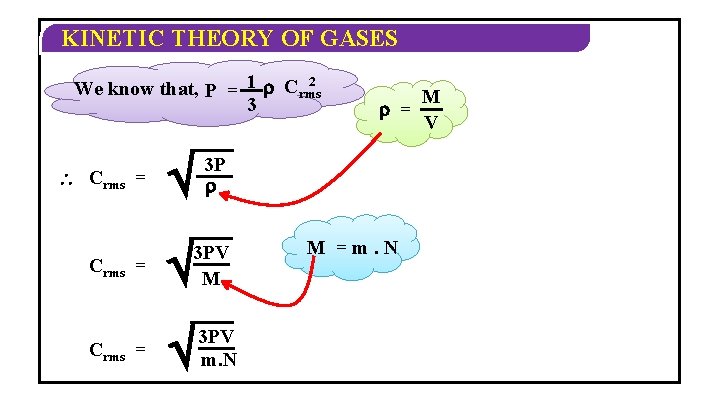
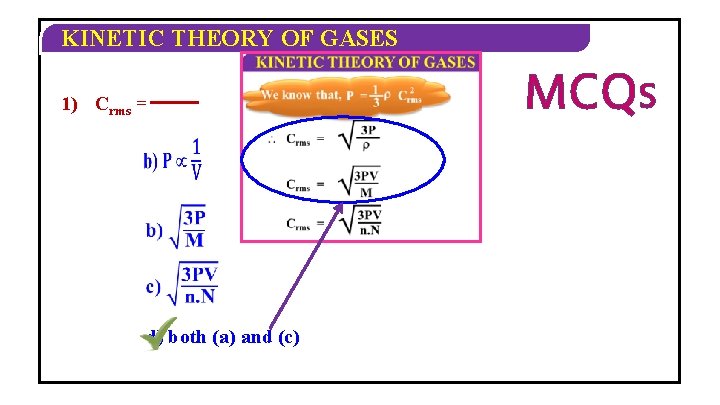
KINETIC THEORY OF GASES 2 We know that, P = 1 Crms 3 Crms = = 3 P 3 PV M 3 PV m. N M =m. N M V

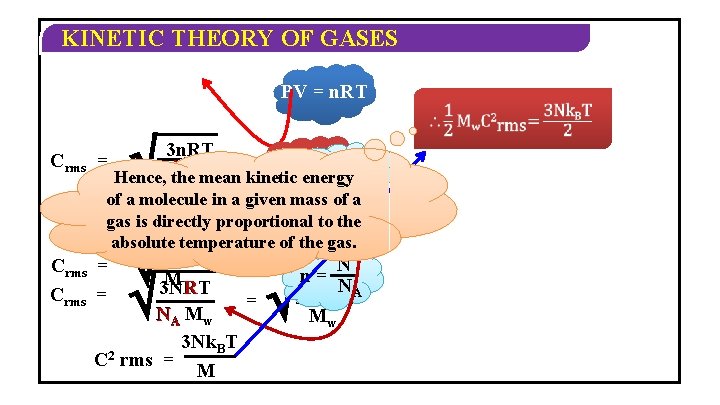
KINETIC THEORY OF GASES PV = n. RT Crms = 3 n. RT M n= R Hence, the M mean kinetic energy NMAw= k. B of a molecule in a given mass of a gas is directly to the 3 RT 3 MRTproportional = absolute temperature = of the gas. M M M Crms = 3 PV 3 NRT MR N M w A C 2 w 3 Nk. BT rms = M N n= 3 Nk N T = M w B A w

KINETIC THEORY OF GASES 1) Crms = d) both (a) and (c) MCQS

KINETIC THEORY OF GASES 2) Crms = ……. d) both (b) and (c) MCQS

KINETIC THEORY OF GASES Thank you…
 Kinetic theory for ideal gases
Kinetic theory for ideal gases Kenetic particle theory
Kenetic particle theory Kinetic theory of gases
Kinetic theory of gases Postulates of kinetic theory of gases class 11
Postulates of kinetic theory of gases class 11 Kinetic theory
Kinetic theory Write the postulates of kinetic theory of gases
Write the postulates of kinetic theory of gases Write postulates of kinetic theory of gases
Write postulates of kinetic theory of gases Kinetic theory of gases
Kinetic theory of gases Molecular theory of gases and liquids
Molecular theory of gases and liquids Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids The kinetic theory of matter states that
The kinetic theory of matter states that Kinetic theory of matter definition
Kinetic theory of matter definition Kinetic theory of matter
Kinetic theory of matter The kinetic theory explains how particles in matter behave
The kinetic theory explains how particles in matter behave Kinetic molecular theory volume
Kinetic molecular theory volume Adhesive force
Adhesive force Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Postulates of kinetic molecular theory
Postulates of kinetic molecular theory Kinetic theory def
Kinetic theory def Kinetic molecular theory timeline
Kinetic molecular theory timeline Kinetic theory of solids
Kinetic theory of solids Charles law in terms of kinetic molecular theory
Charles law in terms of kinetic molecular theory Kmt law
Kmt law What is a kinetic theory of matter
What is a kinetic theory of matter Pv=1/3nmc^2
Pv=1/3nmc^2 Kinetic molecular theory
Kinetic molecular theory Tenets of kinetic molecular theory
Tenets of kinetic molecular theory Cual es la constante universal de los gases
Cual es la constante universal de los gases What is boron
What is boron Expansion of solids liquids and gases examples
Expansion of solids liquids and gases examples Periodic table metals nonmetals metalloids noble gases
Periodic table metals nonmetals metalloids noble gases Colour of noble gases
Colour of noble gases Greenhouse gases are good or bad
Greenhouse gases are good or bad Characteristics of gases
Characteristics of gases Atmosphere it is a mixture of
Atmosphere it is a mixture of Buoyancyability
Buoyancyability Stoichiometry of gases
Stoichiometry of gases Venn diagram states of matter
Venn diagram states of matter The actual exchange of gases occurs at the site of the
The actual exchange of gases occurs at the site of the