Gases Kinetic Molecular Theory 1 Gases Pushing gas




- Slides: 24

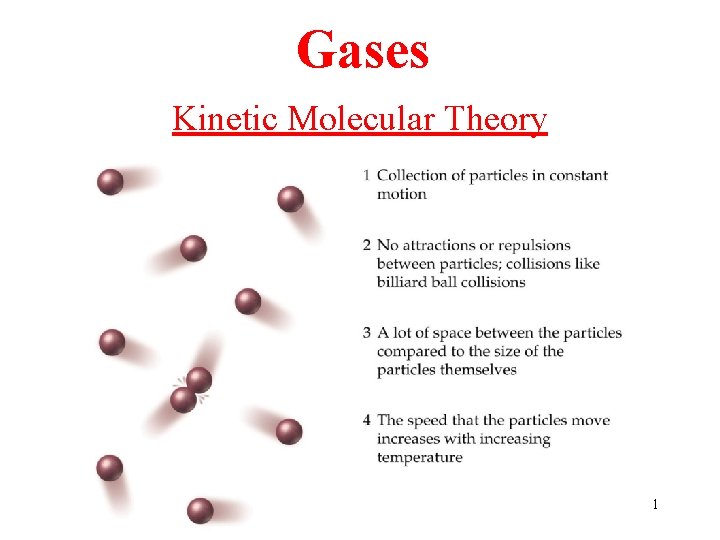
Gases Kinetic Molecular Theory 1

Gases Pushing • gas molecules are constantly in motion • as they move and strike a surface, they push on that surface ü push = force • if we could measure the total amount of force exerted by gas molecules hitting the entire surface at any one instant, we would know the pressure _______ the gas is exerting ü pressure = force per unit area 2

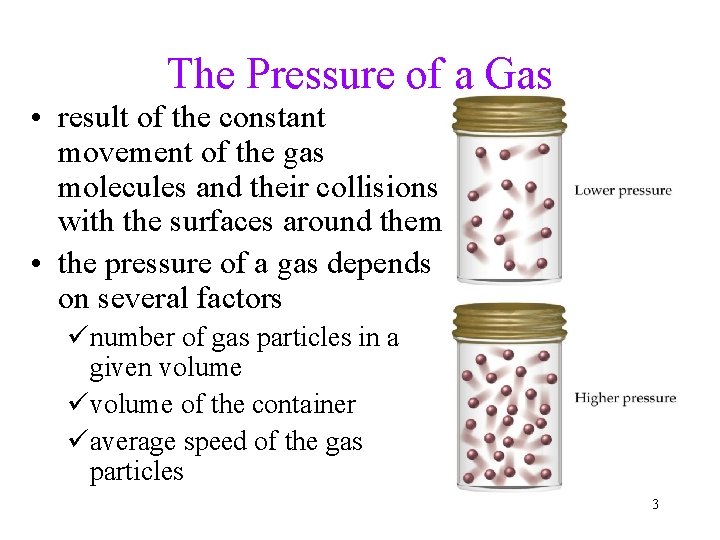
The Pressure of a Gas • result of the constant movement of the gas molecules and their collisions with the surfaces around them • the pressure of a gas depends on several factors ünumber of gas particles in a given volume üvolume of the container üaverage speed of the gas particles 3

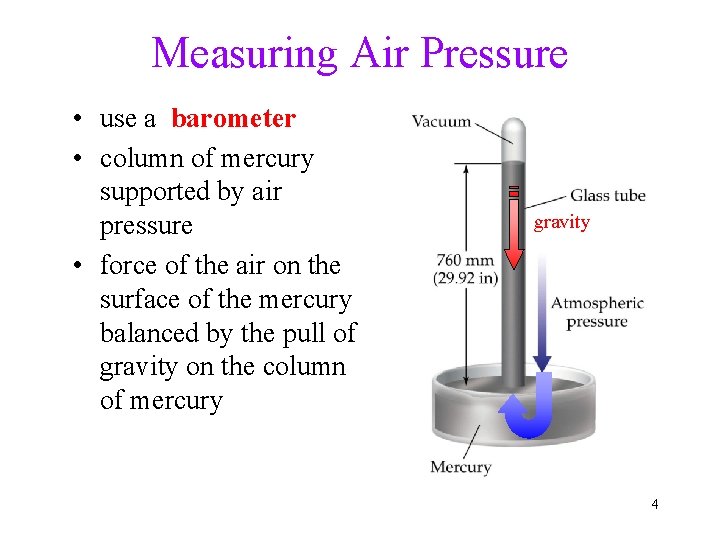
Measuring Air Pressure • use a barometer • column of mercury supported by air pressure • force of the air on the surface of the mercury balanced by the pull of gravity on the column of mercury gravity 4

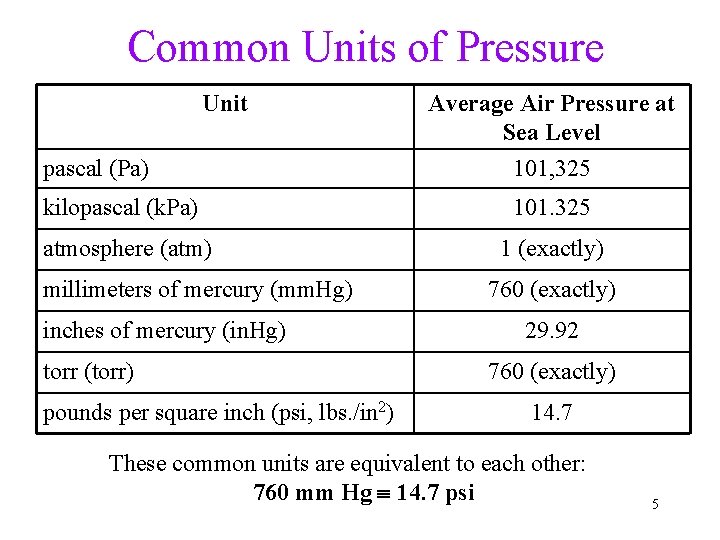
Common Units of Pressure Unit pascal (Pa) Average Air Pressure at Sea Level 101, 325 kilopascal (k. Pa) 101. 325 atmosphere (atm) 1 (exactly) millimeters of mercury (mm. Hg) inches of mercury (in. Hg) torr (torr) pounds per square inch (psi, lbs. /in 2) 760 (exactly) 29. 92 760 (exactly) 14. 7 These common units are equivalent to each other: 760 mm Hg 14. 7 psi 5

Example: Converting Between Pressure Units • A high-performance road bicycle is inflated to a total pressure of 125 psi. What is the pressure in millimeters of mercury? • Sig. Figs. & Round: = 6. 46259 x 103 mm. Hg = 6. 46 x 103 mm. Hg the 760 is an exact number and does not effect the significant figures 6

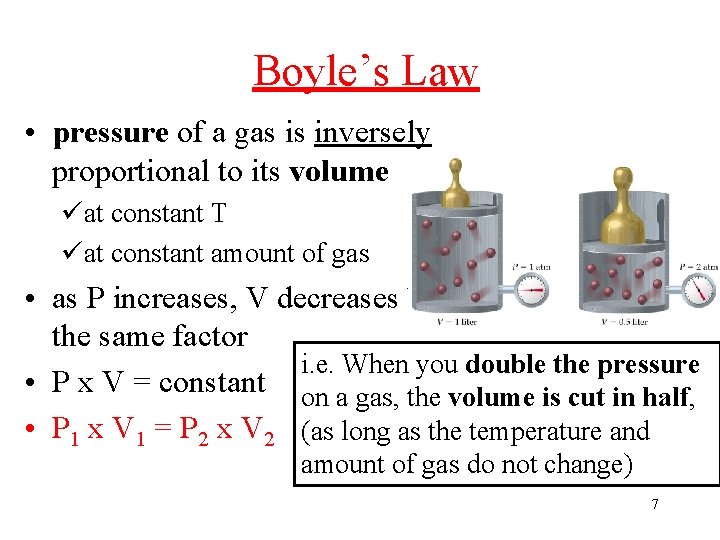
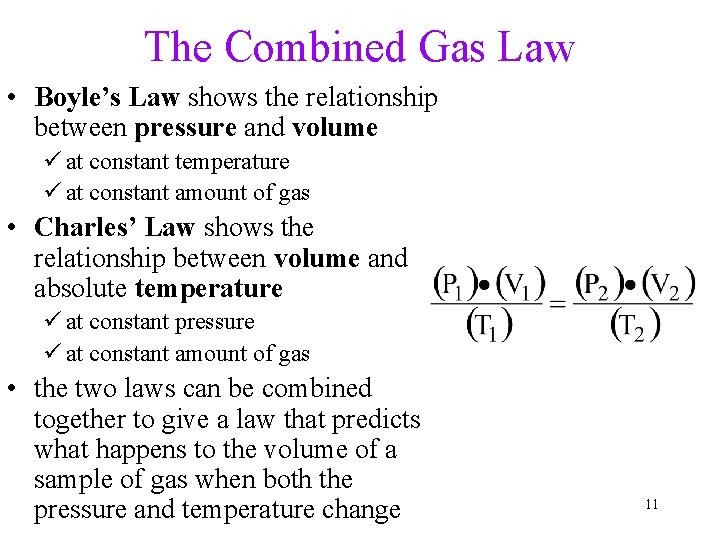
Boyle’s Law • pressure of a gas is inversely proportional to its volume üat constant T üat constant amount of gas • as P increases, V decreases by the same factor i. e. When you double the pressure • P x V = constant on a gas, the volume is cut in half, • P 1 x V 1 = P 2 x V 2 (as long as the temperature and amount of gas do not change) 7

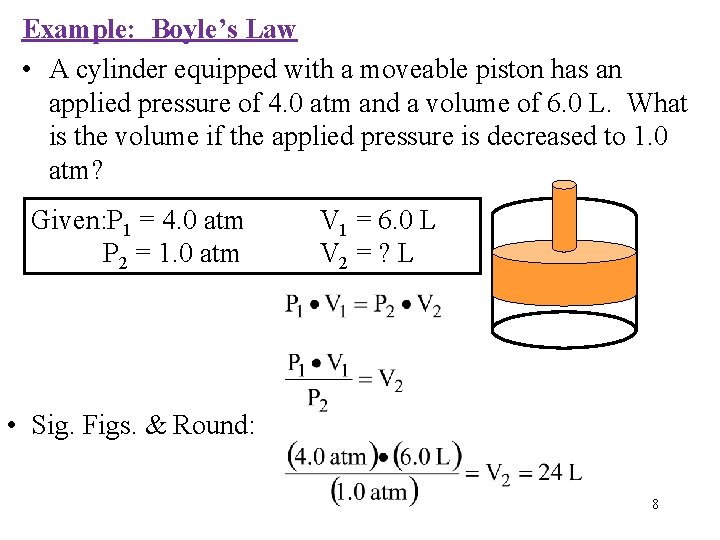
Example: Boyle’s Law • A cylinder equipped with a moveable piston has an applied pressure of 4. 0 atm and a volume of 6. 0 L. What is the volume if the applied pressure is decreased to 1. 0 atm? Given: P 1 = 4. 0 atm P 2 = 1. 0 atm V 1 = 6. 0 L V 2 = ? L • Sig. Figs. & Round: 8

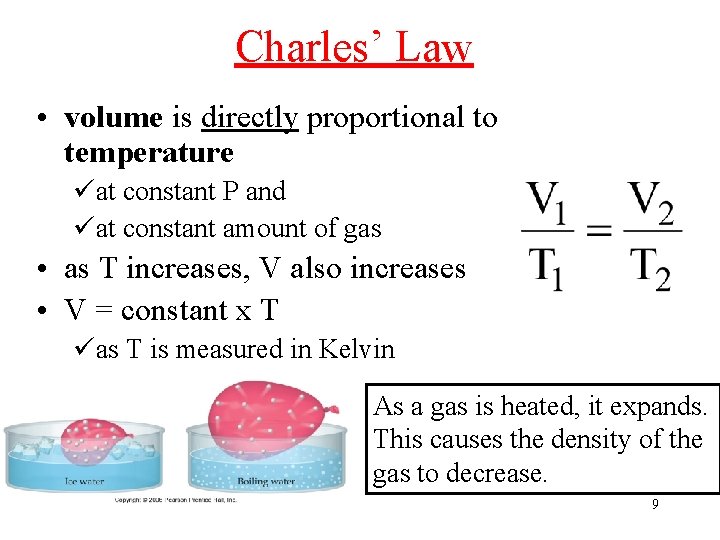
Charles’ Law • volume is directly proportional to temperature üat constant P and üat constant amount of gas • as T increases, V also increases • V = constant x T üas T is measured in Kelvin As a gas is heated, it expands. This causes the density of the gas to decrease. 9

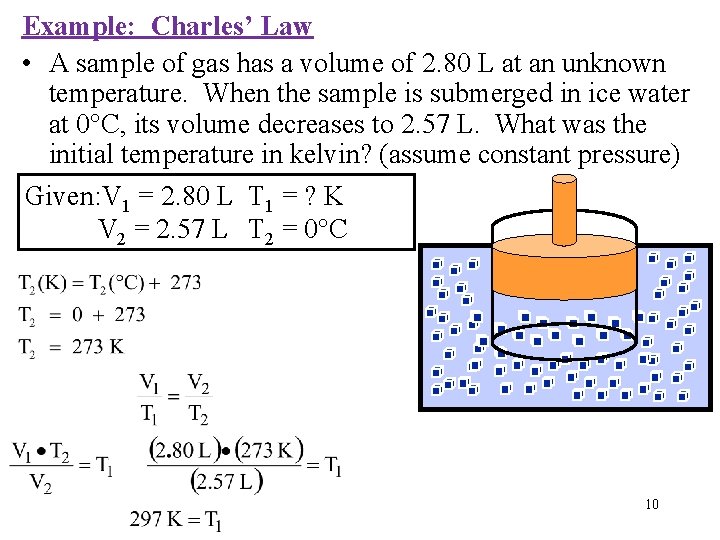
Example: Charles’ Law • A sample of gas has a volume of 2. 80 L at an unknown temperature. When the sample is submerged in ice water at 0°C, its volume decreases to 2. 57 L. What was the initial temperature in kelvin? (assume constant pressure) Given: V 1 = 2. 80 L T 1 = ? K V 2 = 2. 57 L T 2 = 0°C 10

The Combined Gas Law • Boyle’s Law shows the relationship between pressure and volume ü at constant temperature ü at constant amount of gas • Charles’ Law shows the relationship between volume and absolute temperature ü at constant pressure ü at constant amount of gas • the two laws can be combined together to give a law that predicts what happens to the volume of a sample of gas when both the pressure and temperature change 11

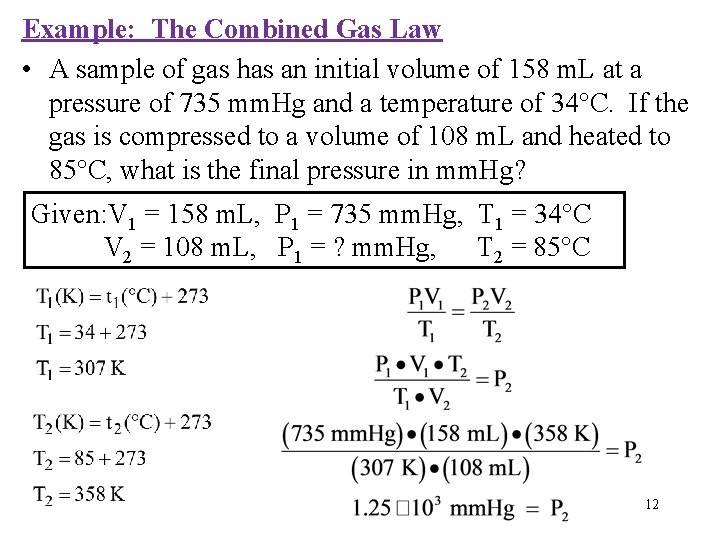
Example: The Combined Gas Law • A sample of gas has an initial volume of 158 m. L at a pressure of 735 mm. Hg and a temperature of 34°C. If the gas is compressed to a volume of 108 m. L and heated to 85°C, what is the final pressure in mm. Hg? Given: V 1 = 158 m. L, P 1 = 735 mm. Hg, T 1 = 34°C V 2 = 108 m. L, P 1 = ? mm. Hg, T 2 = 85°C 12

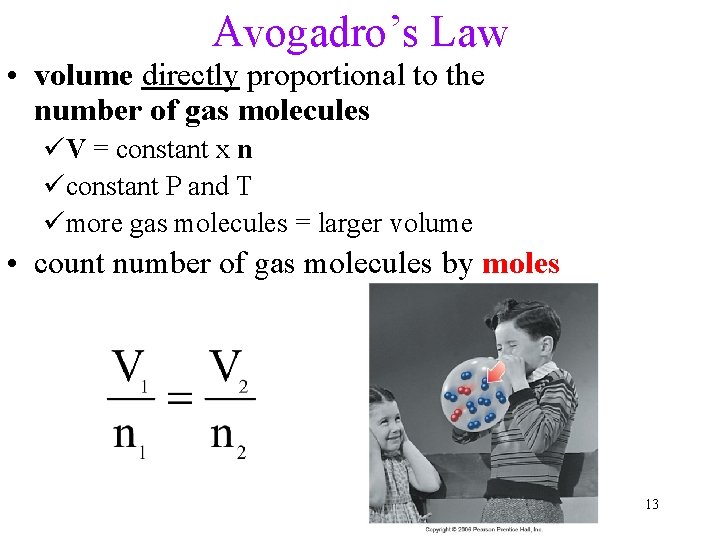
Avogadro’s Law • volume directly proportional to the number of gas molecules üV = constant x n üconstant P and T ümore gas molecules = larger volume • count number of gas molecules by moles 13

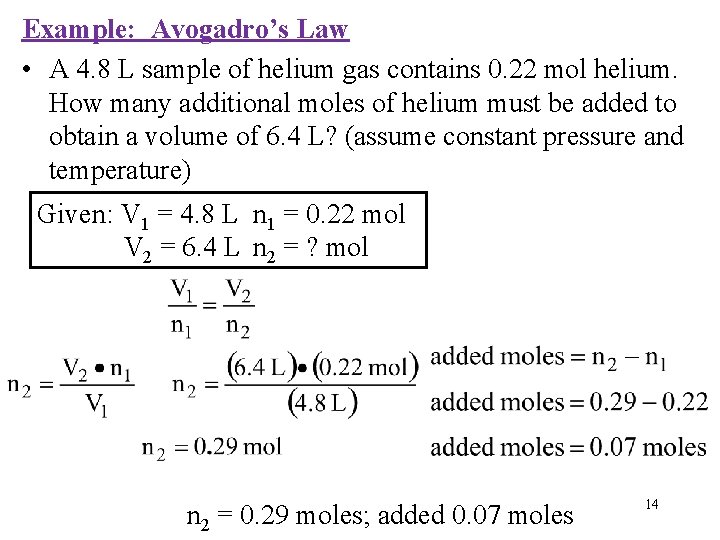
Example: Avogadro’s Law • A 4. 8 L sample of helium gas contains 0. 22 mol helium. How many additional moles of helium must be added to obtain a volume of 6. 4 L? (assume constant pressure and temperature) Given: V 1 = 4. 8 L n 1 = 0. 22 mol V 2 = 6. 4 L n 2 = ? mol n 2 = 0. 29 moles; added 0. 07 moles 14

Ideal Gas Law • By combing the gas laws we can write a general equation: • R is called the Gas Constant • the value of R depends on the units of P and V ü but we will use 0. 0821 ü therefore we need to keep P in atm and V in L • use the Ideal Gas law when we have a gas at one condition, use the Combined Gas Law when you have gas whose condition is changing 15

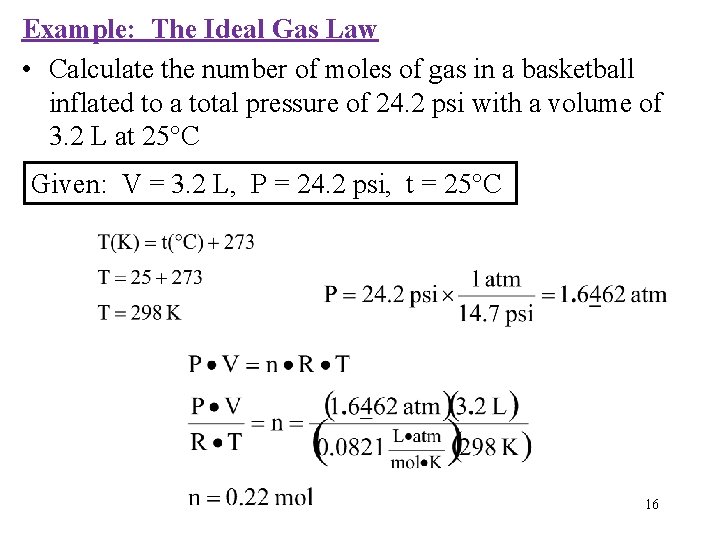
Example: The Ideal Gas Law • Calculate the number of moles of gas in a basketball inflated to a total pressure of 24. 2 psi with a volume of 3. 2 L at 25°C Given: V = 3. 2 L, P = 24. 2 psi, t = 25°C 16

Ideal vs. Real 17

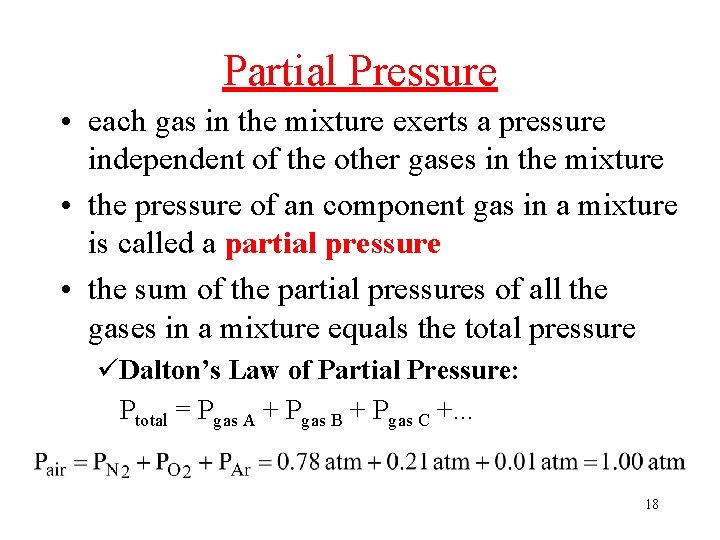
Partial Pressure • each gas in the mixture exerts a pressure independent of the other gases in the mixture • the pressure of an component gas in a mixture is called a partial pressure • the sum of the partial pressures of all the gases in a mixture equals the total pressure üDalton’s Law of Partial Pressure: Ptotal = Pgas A + Pgas B + Pgas C +. . . 18

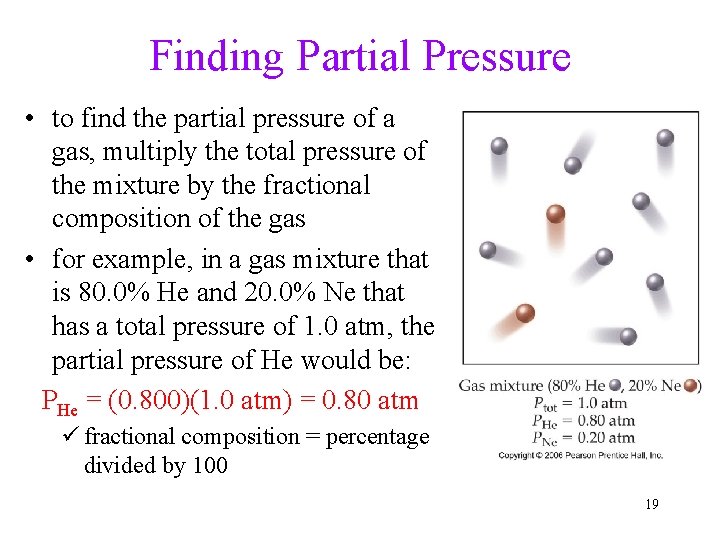
Finding Partial Pressure • to find the partial pressure of a gas, multiply the total pressure of the mixture by the fractional composition of the gas • for example, in a gas mixture that is 80. 0% He and 20. 0% Ne that has a total pressure of 1. 0 atm, the partial pressure of He would be: PHe = (0. 800)(1. 0 atm) = 0. 80 atm ü fractional composition = percentage divided by 100 19

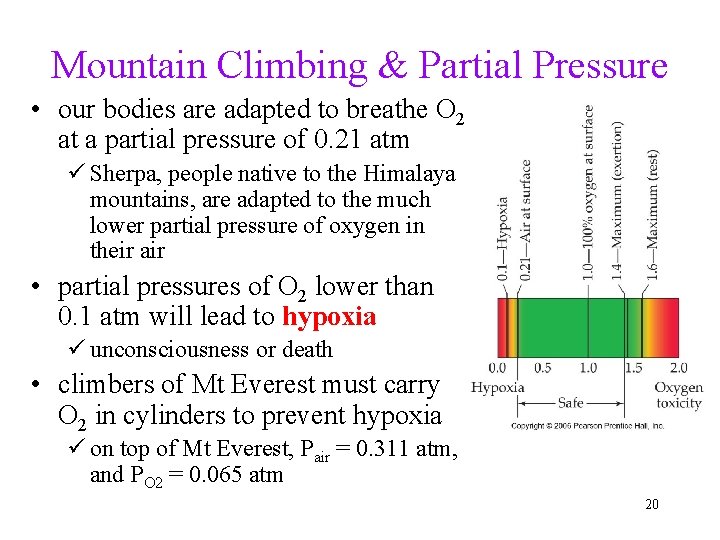
Mountain Climbing & Partial Pressure • our bodies are adapted to breathe O 2 at a partial pressure of 0. 21 atm ü Sherpa, people native to the Himalaya mountains, are adapted to the much lower partial pressure of oxygen in their air • partial pressures of O 2 lower than 0. 1 atm will lead to hypoxia ü unconsciousness or death • climbers of Mt Everest must carry O 2 in cylinders to prevent hypoxia ü on top of Mt Everest, Pair = 0. 311 atm, and PO 2 = 0. 065 atm 20

Standard Conditions • Common reference points for comparing Standard Temperature & Pressure • STP (_______________) üstandard pressure = 1. 00 atm üstandard temperature = 0°C Ø 273 K 21

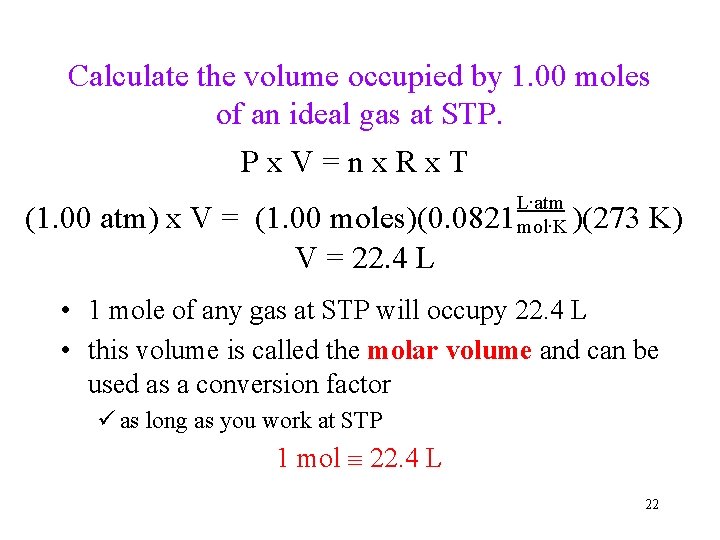
Calculate the volume occupied by 1. 00 moles of an ideal gas at STP. Px. V=nx. Rx. T (1. 00 atm) x V = (1. 00 moles)(0. 0821 V = 22. 4 L L∙atm mol∙K )(273 K) • 1 mole of any gas at STP will occupy 22. 4 L • this volume is called the molar volume and can be used as a conversion factor ü as long as you work at STP 1 mol 22. 4 L 22

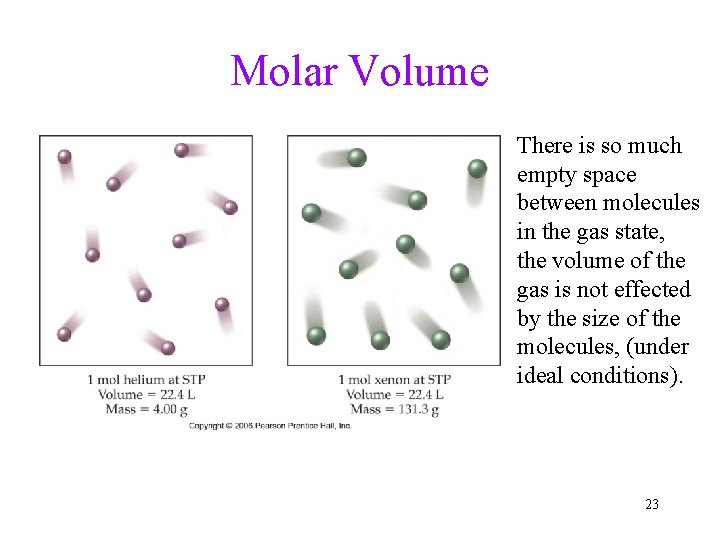
Molar Volume There is so much empty space between molecules in the gas state, the volume of the gas is not effected by the size of the molecules, (under ideal conditions). 23

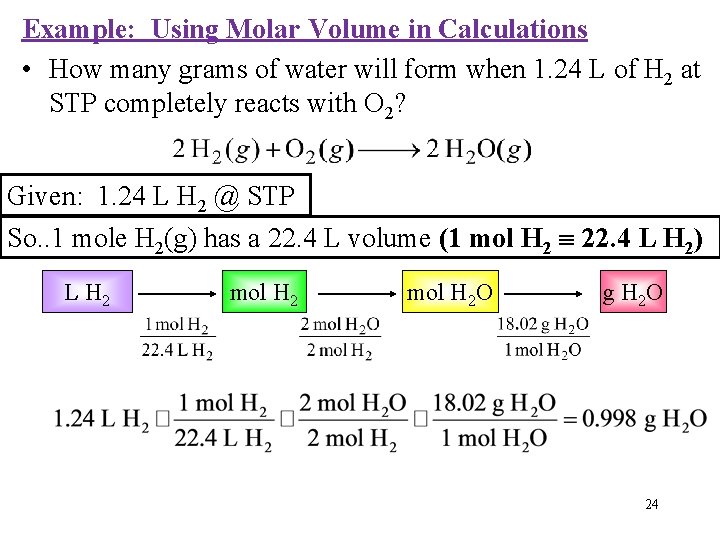
Example: Using Molar Volume in Calculations • How many grams of water will form when 1. 24 L of H 2 at STP completely reacts with O 2? Given: 1. 24 L H 2 @ STP So. . 1 mole H 2(g) has a 22. 4 L volume (1 mol H 2 22. 4 L H 2) L H 2 mol H 2 O g H 2 O 24
 Kinetic theory for ideal gases
Kinetic theory for ideal gases Book
Book Kinetic molecular theory of solids
Kinetic molecular theory of solids Kinetic molecular theory volume
Kinetic molecular theory volume Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Kinetic energy molecular theory
Kinetic energy molecular theory Kinetic molecular theory def
Kinetic molecular theory def Kinetic molecular theory timeline
Kinetic molecular theory timeline Charles law in terms of kinetic molecular theory
Charles law in terms of kinetic molecular theory Kinetic theory postulates
Kinetic theory postulates Kinetic molecular theory
Kinetic molecular theory Kinetic molecular theory
Kinetic molecular theory Kinetic molecular theory formula
Kinetic molecular theory formula Kinetic molecular theory
Kinetic molecular theory Tenets of kinetic molecular theory
Tenets of kinetic molecular theory Particle theory of freezing
Particle theory of freezing Kinetic theory of gases
Kinetic theory of gases Kinetic theory of gases
Kinetic theory of gases Write postulates of kinetic theory of gases
Write postulates of kinetic theory of gases Kinetic theory
Kinetic theory Molecular theory of gases and liquids
Molecular theory of gases and liquids Vbt vs mot
Vbt vs mot Px and dxz overlap
Px and dxz overlap Valence bond theory and molecular orbital theory
Valence bond theory and molecular orbital theory