Unit 12 Gas Laws The Kinetic Theory of


































- Slides: 53

Unit 12: Gas Laws

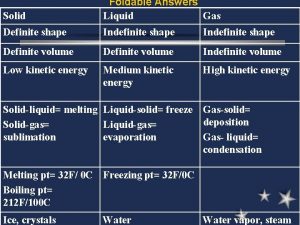
The Kinetic Theory of Gases • Assumes the following statements about gas behavior: – Do not attract or repel each other. This is because they are so spread out. – The distance between one particle and another is much smaller. Therefore, almost all of the volume of a gas is empty space. – Are in constant, random motion. – No kinetic energy is lost when gas particles collide with each other or the sides of their container. These collisions are called elastic. – All gases have the same average kinetic energy at a given temperature. As temperature increases, energy increases.

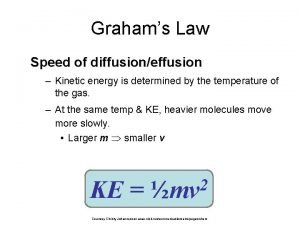
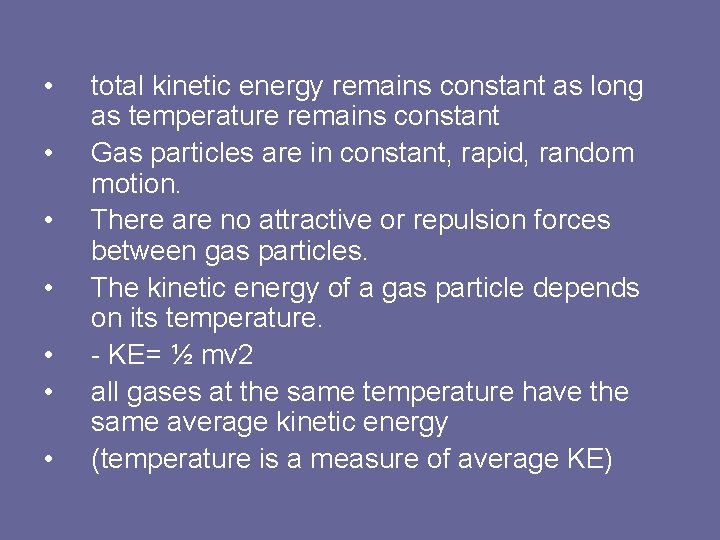
• • total kinetic energy remains constant as long as temperature remains constant Gas particles are in constant, rapid, random motion. There are no attractive or repulsion forces between gas particles. The kinetic energy of a gas particle depends on its temperature. - KE= ½ mv 2 all gases at the same temperature have the same average kinetic energy (temperature is a measure of average KE)

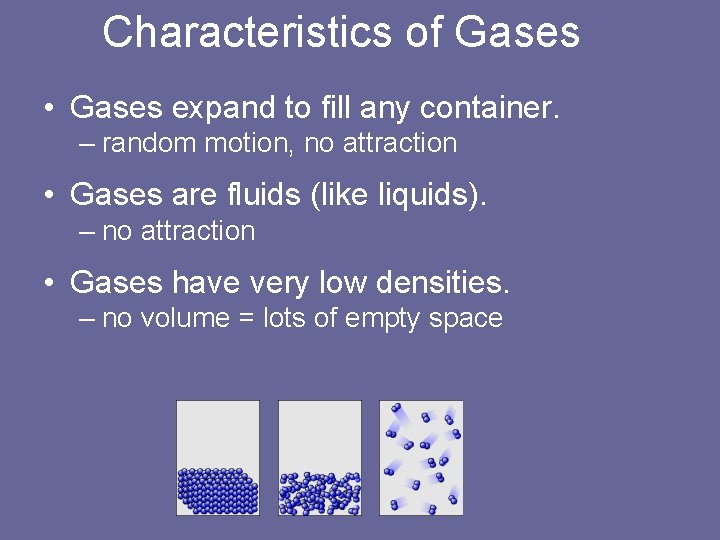
Characteristics of Gases • Gases expand to fill any container. – random motion, no attraction • Gases are fluids (like liquids). – no attraction • Gases have very low densities. – no volume = lots of empty space

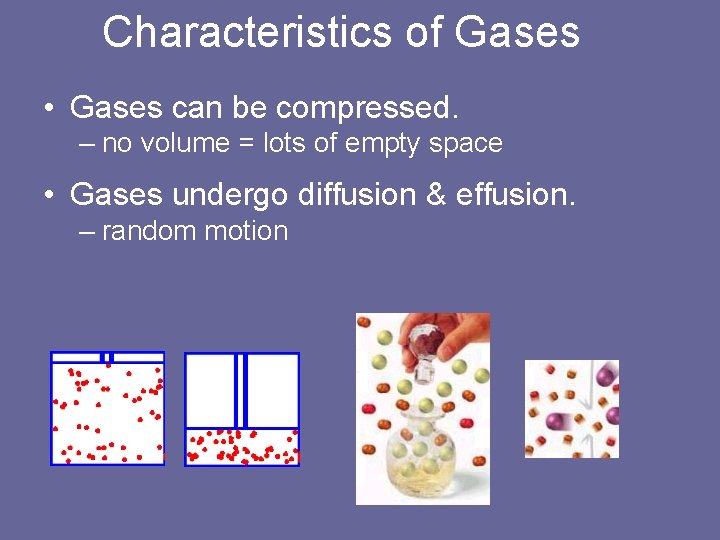
Characteristics of Gases • Gases can be compressed. – no volume = lots of empty space • Gases undergo diffusion & effusion. – random motion

C. Deviations from Ideal Gases • most gases do not exhibit ideal gas behavior • a real gas is a gas that does not behave totally according to the kinetic-molecular theory • most gas particles do exert attractive forces on one another • the deviation from ideal gas behavior is greatest at high pressures and low temperatures. • gases get closer to exhibiting ideal behavior when there is minimal attraction between gas particles (i. e. low pressures and high temperatures)

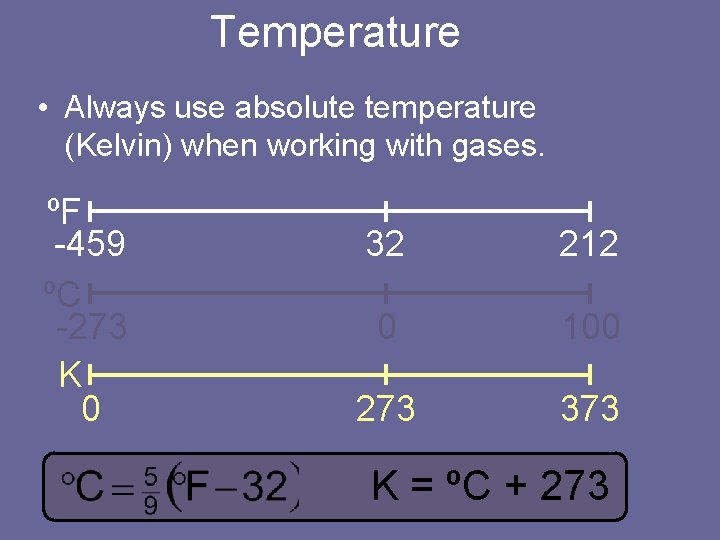
Temperature • Always use absolute temperature (Kelvin) when working with gases. ºF -459 ºC -273 K 0 32 212 0 100 273 373 K = ºC + 273

II. Measuring Gases A. Temperature • Temperature is a measure of the average kinetic energy of the particles in a material. • The average kinetic energy of a gas is directly proportional to its temperature.

Important Temperatures Absolute Zero* Freezing point of water Standard Temperature Boiling point of water °C K

Pressure Which shoes create the most pressure?

Atmospheric Pressure • The earth is surrounded by an atmosphere that extends for hundreds of kilometers. This blanket of air is pushing down on us at all times. At sea level, the pressure is equal to 14. 7 pounds per square inch. (pounds is the force unit, square inch is the area unit) • Atmospheric pressure is measured with a barometer. The barometer was invented by Evangelista Torricelli (1608 -1647). Torricelli said, “We live at the bottom of an ocean of air. ”

Torricelli’s barometer:

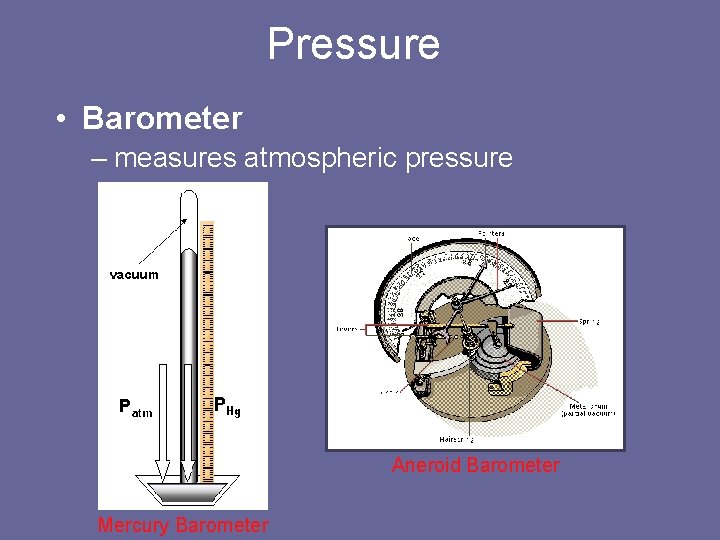
Pressure • Barometer – measures atmospheric pressure Aneroid Barometer Mercury Barometer

• Pressure varies with altitude. The higher the altitude, the lower the atmospheric pressure, because there is less atmosphere piled on top of you if you are higher on the earth’s surface. Also, the air tends to be “thinner” at higher altitudes. • Examples: Location Elevation Atmospheric Pressure in pounds per square inch (psi) Galveston, TX Sea level 14. 7 psi Denver, CO 5280 ft 12. 2 psi Pike’s Peak, CO 14, 000 ft 8. 8 psi Top of Mt. Everest 29, 000 ft 4. 9 psi

Pressure Units and Conversions Pressure units can look a bit strange. Here is an explanation of the ones you will most commonly use: • Pounds per square inch = psi; simply expresses the force in pounds over an area in inches 2 • Atmosphere = atm; 1 atm is the pressure at sea level (the entire atmosphere is pushing down) • Millimeters of mercury or inches of mercury = mm Hg or in Hg; comes from the height that Hg climbs in a barometer • Torr; named after Torricelli and is equal to mm Hg • Pascal = Pa; named after Blaise Pascal, a famous scientist who studied gas pressure

Conversions • Kilopascal = k. Pa; just 1000 times greater than a Pascal • Relationships between pressure units: **important for conversions!!! 1 atm = 14. 7 psi = 760 mm Hg = 29. 9 in Hg = 760 Torr = 101, 300 Pa = 101. 3 k. Pa

Try these pressure conversions: • 1. 0. 50 atm = ? k. Pa • 2. 744 Torr = ? mm Hg • 3. 17. 9 psi = ? Atm • 4. 155 k. Pa = ? psi

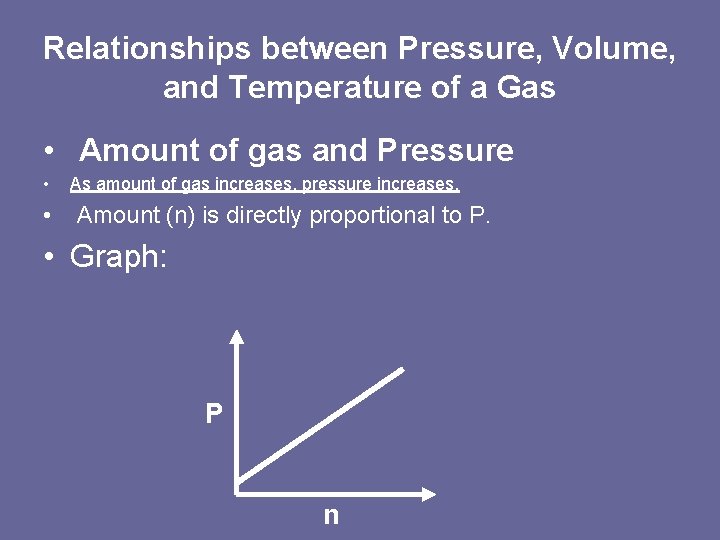
Relationships between Pressure, Volume, and Temperature of a Gas • Amount of gas and Pressure • • As amount of gas increases, pressure increases. Amount (n) is directly proportional to P. • Graph: P n

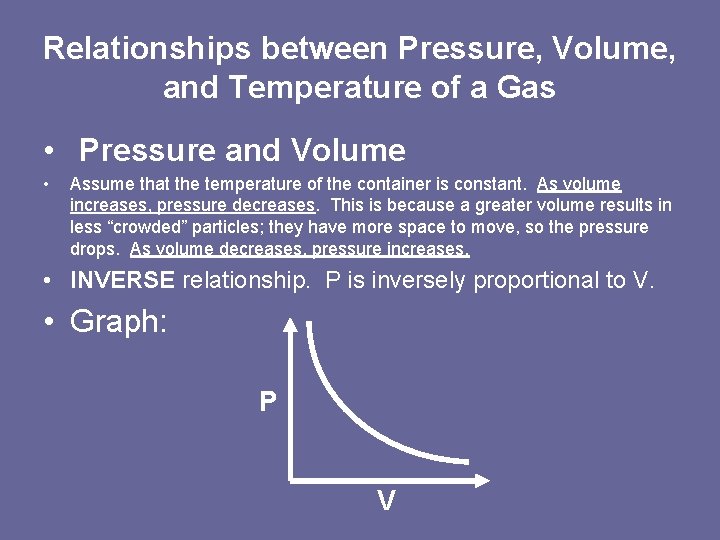
Relationships between Pressure, Volume, and Temperature of a Gas • Pressure and Volume • Assume that the temperature of the container is constant. As volume increases, pressure decreases. This is because a greater volume results in less “crowded” particles; they have more space to move, so the pressure drops. As volume decreases, pressure increases. • INVERSE relationship. P is inversely proportional to V. • Graph: P V

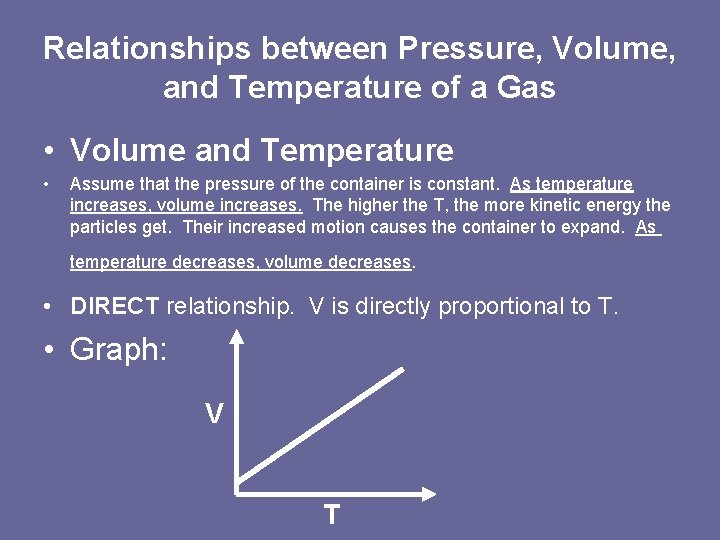
Relationships between Pressure, Volume, and Temperature of a Gas • Volume and Temperature • Assume that the pressure of the container is constant. As temperature increases, volume increases. The higher the T, the more kinetic energy the particles get. Their increased motion causes the container to expand. As temperature decreases, volume decreases. • DIRECT relationship. V is directly proportional to T. • Graph: V T

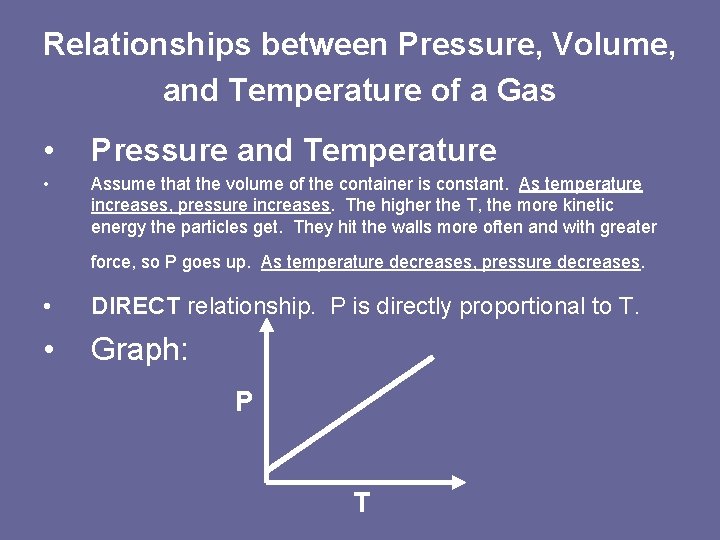
Relationships between Pressure, Volume, and Temperature of a Gas • Pressure and Temperature • Assume that the volume of the container is constant. As temperature increases, pressure increases. The higher the T, the more kinetic energy the particles get. They hit the walls more often and with greater force, so P goes up. As temperature decreases, pressure decreases. • DIRECT relationship. P is directly proportional to T. • Graph: P T

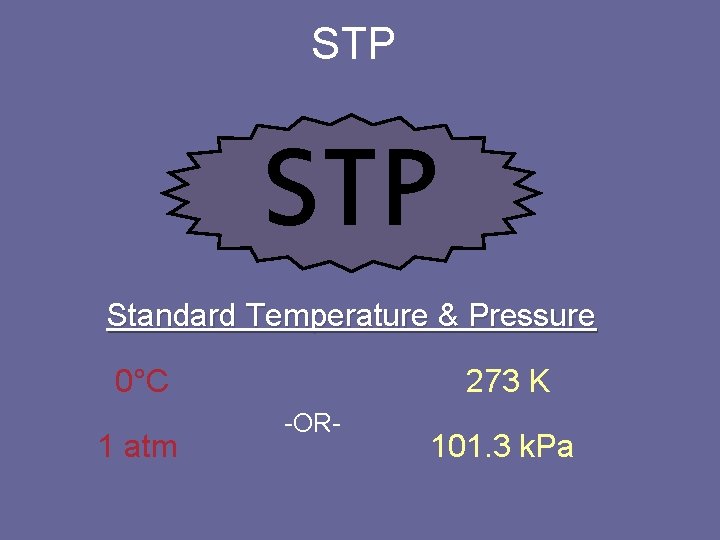
STP Standard Temperature & Pressure 0°C 1 atm 273 K -OR- 101. 3 k. Pa

Boyle’s Law • The pressure and volume of a gas are inversely related – at constant mass & temp P 1 V 1 =P 2 V 2 P V

Example: • A 5. 0 L container of nitrogen gas is at a pressure of 1. 0 atm. What is the new pressure if the volume is decreased to 500 m. L, and the temperature remains constant?

Charles’ Law • The volume and absolute temperature (K) of a gas are directly related – at constant mass & pressure V T

Example: • A container of helium gas at 25 o. C in an expandable 500 m. L container is heated to 80. o. C. What is the new volume if the pressure remains constant?

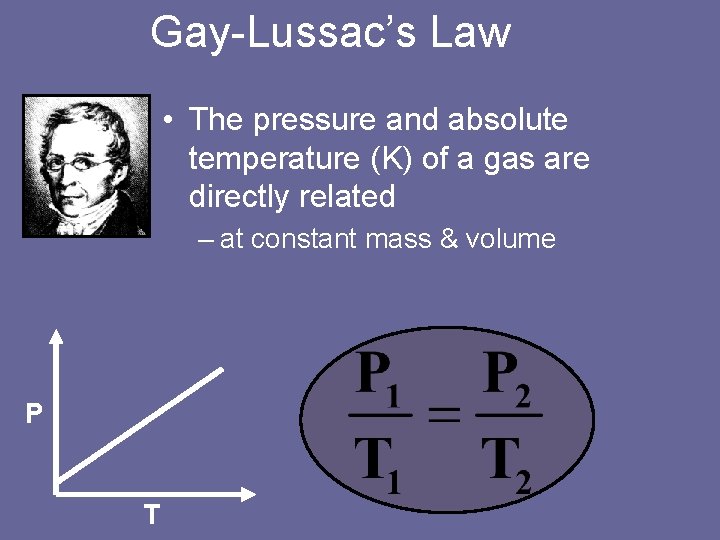
Gay-Lussac’s Law • The pressure and absolute temperature (K) of a gas are directly related – at constant mass & volume P T

Example: • A tank of propane gas at a pressure of 3. 0 atm is cooled from 90. o. C to 30. o. C. What is the new pressure if the volume remains constant?

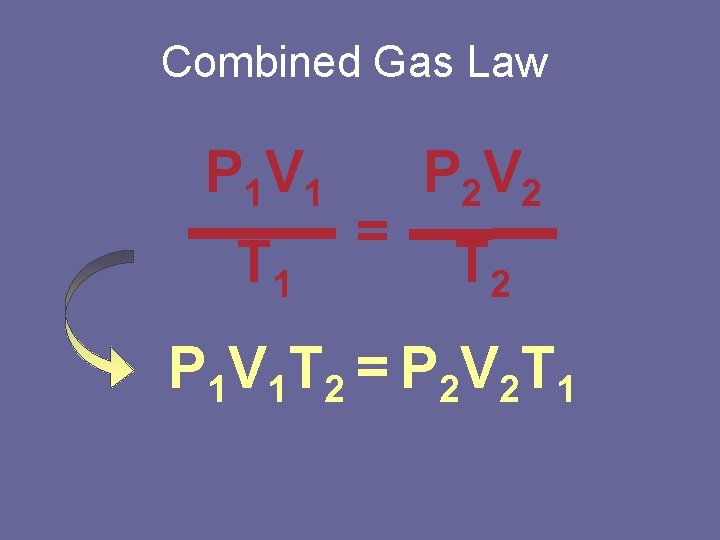
Combined Gas Law P 1 V 1 T 1 = P 2 V 2 T 2 P 1 V 1 T 2 = P 2 V 2 T 1

Example: • A high-altitude balloon contains 30. 0 L of helium at 103 k. Pa. What is the volume when the balloon rises to an altitude where the pressure is only 25. 0 k. Pa? (Assume constant n and T)

Dalton’s Law of Partial Pressures • The total pressure of a mixture of gases is equal to the sum of the partial pressures of the component gases. • In a mixture of gases, the total pressure is the sum of the partial pressures. Ptotal = P 1 + P 2 + P 3 + …

Example • A gas mixture containing oxygen, nitrogen, and carbon dioxide has a total pressure of 257 mm Hg. If PO 2 = 52 mm Hg and PN 2= 171 mm Hg, what is PCO 2? Ptotal = PO 2 + PN 2 + PCO 2 257 mm. Hg = 52 mm. Hg + 171 mm. Hg + PCO 2 = 34 mm. Hg

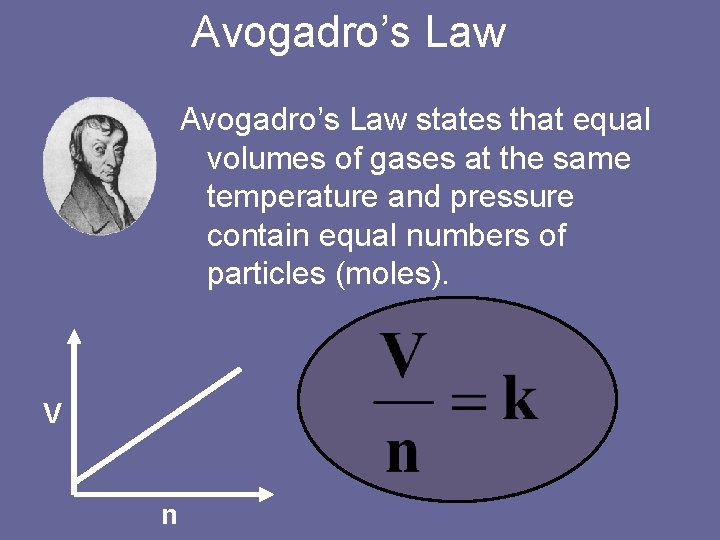
Avogadro’s Law states that equal volumes of gases at the same temperature and pressure contain equal numbers of particles (moles). V n

Avogadro’s Law • Avogadro’s Law says that equal volumes of gases at the same temperature and pressure contain equal numbers of moles. • This means that the coefficients in the balanced equation stand for volume as well as moles, but only for the gases. Remember, at STP, 1 mole of any gas will have a volume of 22. 4 L.

Ideal Gas Law • The ideal gas law is different from the previous laws in that: – it is not used for “initial and final conditions” problems. – it only uses one set of conditions – it incorporates the moles of the gas, represented by n

A. Ideal Gas Law PV=n. RT UNIVERSAL GAS CONSTANT R=0. 0821 L atm/mol K

YOU MUST CONVERT: • P into atm – Use pressure conversions • V into L – Divide by 1000 if m. L • n into moles – divide by molecular weight • T into K – Add 273 to Celsius

Example: • You fill a rigid steel cylinder that has a volume of 20. 0 L with nitrogen to a final pressure of 2. 00 x 104 k. Pa at 28°C. How many moles of nitrogen does the cylinder contain?

• for density (D) or molar mass (M) of a gas: – (density has the units g/L; recall that molar mass is in units of g/mol) M = DRT P 1. 67 g of an unknown liquid are vaporized at a temperature of 125 C. The gas volume is measured as 0. 421 L at 749 mm Hg. Calculate the molar mass.

Gas Stoichiometry • Review the 3 basic steps in stoichiometry: • 1) Identify the given, and convert it to moles if not already in moles. • 2) Identify the unknown, and do a mole-to-mole ratio between given and unknown using the coefficients from the balanced equation. This is the key step: it gets you from moles of given to moles of unknown. • 3) Convert the unknown to the unit specified in the problem. If it asks for moles, you are finished at step 2!

A. Gas Stoichiometry • Look at problems, if: – STP - use 22. 4 L/mol – Non-STP - use ideal gas law • Non-STP – Given liters of gas? • start with ideal gas law – Looking for liters of gas? • start with stoichiometry conv.

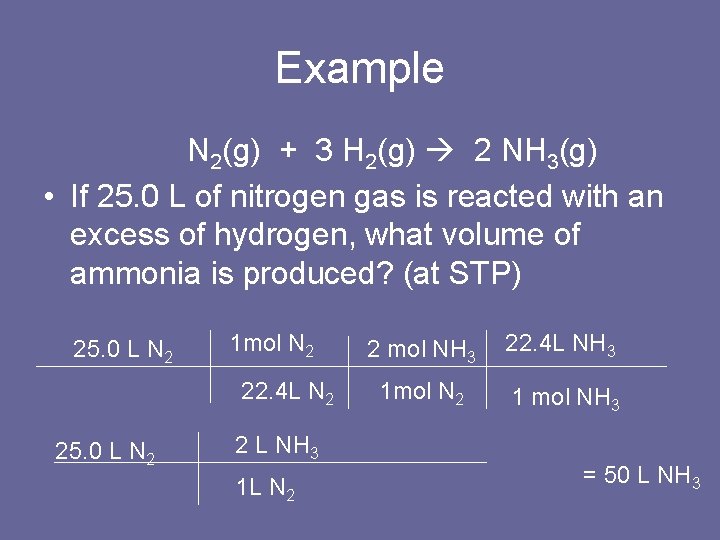
Example N 2(g) + 3 H 2(g) 2 NH 3(g) • If 25. 0 L of nitrogen gas is reacted with an excess of hydrogen, what volume of ammonia is produced? (at STP) 25. 0 L N 2 1 mol N 2 22. 4 L N 2 25. 0 L N 2 2 L NH 3 1 L N 2 2 mol NH 3 22. 4 L NH 3 1 mol N 2 1 mol NH 3 = 50 L NH 3

Gas Stoichiometry Problem • What volume of CO 2 forms from 5. 25 g of Ca. CO 3 at 103 k. Pa & 25ºC? Ca. CO 3 5. 25 g Ca. O + Looking for liters: Start with stoich and calculate moles of CO 2. 5. 25 g 1 mol Ca. CO 3 1 mol CO 2 100. 09 g 1 mol Ca. CO 3 CO 2 ? L non-STP = 1. 26 mol CO 2 Plug this into the Ideal Gas Law to find liters.

Gas Stoichiometry Problem • What volume of CO 2 forms from 5. 25 g of Ca. CO 3 at 103 k. Pa & 25ºC? GIVEN: WORK: P = 103 k. Pa/1. 01 atm V=? n = 1. 26 mol T = 25°C = 298 K R = 0. 0821 L atm/mol K PV = n. RT (1. 01 atm)V =(1. 26 mol)(0. 0821 L atm/mol K)(29 8 K) V = 30. 5 LCO 2

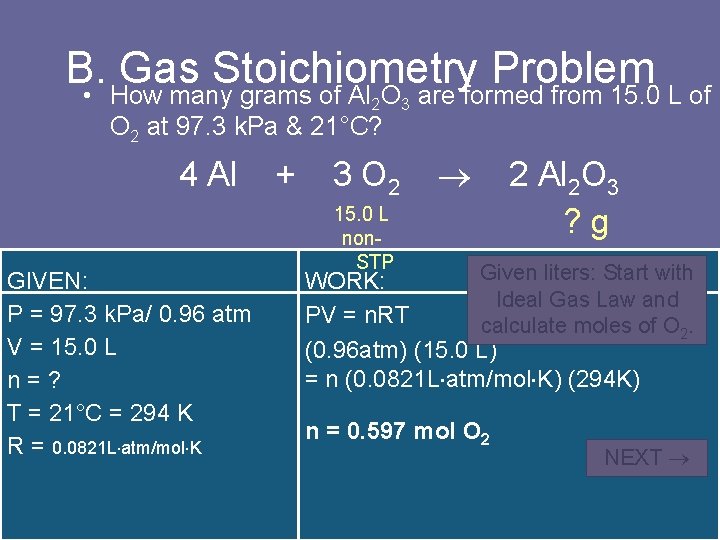
B. Gas Stoichiometry Problem • How many grams of Al O are formed from 15. 0 L of 2 O 2 at 97. 3 k. Pa & 21°C? 4 Al GIVEN: P = 97. 3 k. Pa/ 0. 96 atm V = 15. 0 L n=? T = 21°C = 294 K R = 0. 0821 L atm/mol K + 3 O 2 3 15. 0 L non. STP 2 Al 2 O 3 ? g Given liters: Start with WORK: Ideal Gas Law and PV = n. RT calculate moles of O 2. (0. 96 atm) (15. 0 L) = n (0. 0821 L atm/mol K) (294 K) n = 0. 597 mol O 2 NEXT

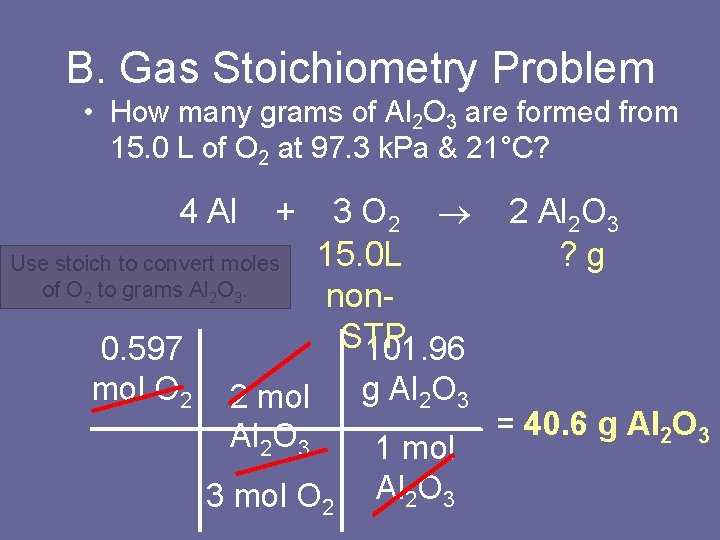
B. Gas Stoichiometry Problem • How many grams of Al 2 O 3 are formed from 15. 0 L of O 2 at 97. 3 k. Pa & 21°C? 4 Al + Use stoich to convert moles of O 2 to grams Al 2 O 3. 0. 597 mol O 2 3 O 2 15. 0 L non. STP 101. 96 2 mol Al 2 O 3 3 mol O 2 g Al 2 O 3 1 mol Al 2 O 3 2 Al 2 O 3 ? g = 40. 6 g Al 2 O 3

Example: N 2 + 2 H 2 2 NH 3 • Of course, typical stoichiometry can be combined with the ideal gas law as well: If 5. 00 L of nitrogen reacts with excess hydrogen at 3. 00 atm and 298 K, what volume of ammonia is produced (at STP)?

Example: 4 Fe(s) + 3 O 2(g) 2 Fe 2 O 3(s) • Refer to the equation above. If 22. 4 L of oxygen gas is reacted at STP (with an excess of iron), how many grams of iron (III) oxide will be formed?

Example 2 H 2(g) + O 2(g) 2 H 2 O(g) • If 300. m. L of water vapor is produced in the above reaction, how many m. L of oxygen reacted?

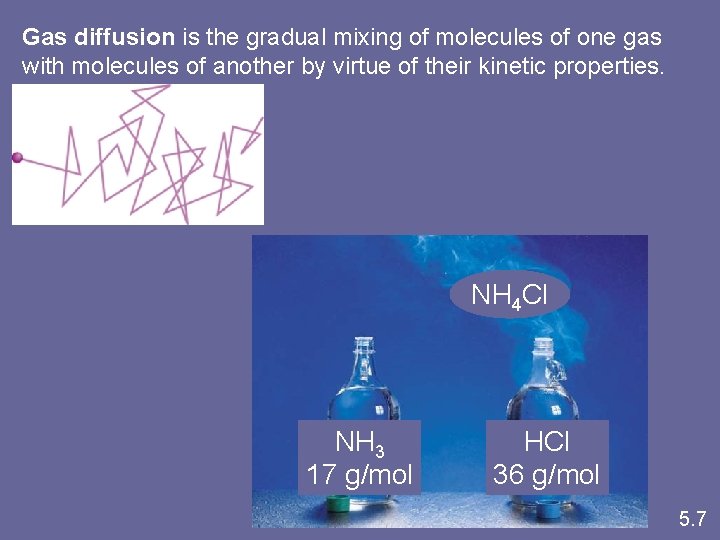
Graham’s Law Diffusion and Effusion • gases will move with one another, without agitation • gradual mixing of gases caused by random motion is called diffusion • rate of diffusion depends on: – their speeds – their diameters – the attractive forces (faster, lighter particles will diffuse more rapidly than slower, heavier particles) • when gas particles pass through a small opening it is called effusion. • rate of effusion is directly proportional to velocities of particles

Gas diffusion is the gradual mixing of molecules of one gas with molecules of another by virtue of their kinetic properties. NH 4 Cl NH 3 17 g/mol HCl 36 g/mol 5. 7

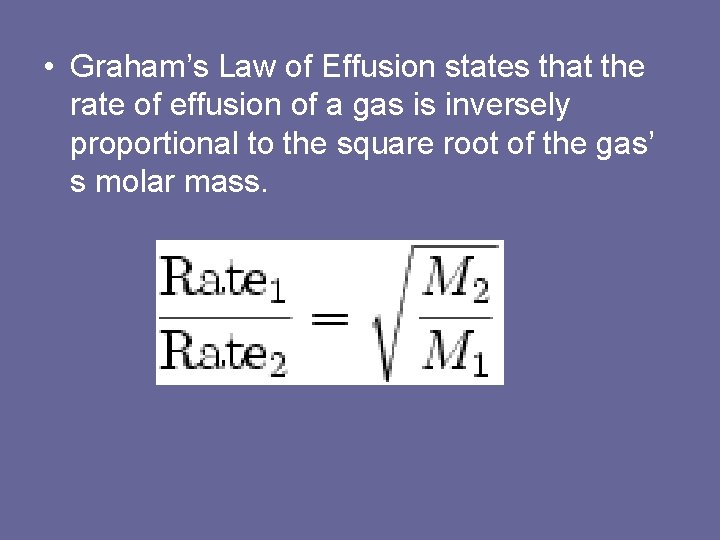
• Graham’s Law of Effusion states that the rate of effusion of a gas is inversely proportional to the square root of the gas’ s molar mass.

Using Graham’s Law: Ex 1: A sample of hydrogen effuses through a porous container about 9 times faster than an unknown gas. Estimate the molar mass of the unknown gas. Ex 2: Calculate the ratio of effusion rates of NO 2 and SO 3 from the same container at the same temperature and pressure.
 Kinetic theory postulates
Kinetic theory postulates Useless laws weaken the necessary laws
Useless laws weaken the necessary laws Relation between pressure and kinetic energy of gas
Relation between pressure and kinetic energy of gas Kinetic energy of gas formula
Kinetic energy of gas formula Www.nisd.net
Www.nisd.net Gas laws crash course
Gas laws crash course Constant direct and indirect relationships
Constant direct and indirect relationships All the gas laws
All the gas laws Combined gas laws formula
Combined gas laws formula Bourdon gauge gas law
Bourdon gauge gas law Different gas laws
Different gas laws Charles law
Charles law Gas law conceptual questions
Gas law conceptual questions Sample problem of boyle's law
Sample problem of boyle's law How to solve ideal gas law
How to solve ideal gas law A gas occupies 473 cm3 at 36°c. find its volume at 94°c
A gas occupies 473 cm3 at 36°c. find its volume at 94°c Different gas laws
Different gas laws Combined gas law worksheet
Combined gas law worksheet Section 13.2 the combined gas law and avogadro's principle
Section 13.2 the combined gas law and avogadro's principle Boyle's law
Boyle's law Charles law formula
Charles law formula Ap chemistry gas laws
Ap chemistry gas laws Gas laws graphic organizer
Gas laws graphic organizer Gas laws hot air balloon
Gas laws hot air balloon Gay lussac's law in real life
Gay lussac's law in real life Kmt gas laws
Kmt gas laws Gas law formulas
Gas law formulas Empirical gas law
Empirical gas law Combined gas laws
Combined gas laws Kpa gas constant
Kpa gas constant Gas laws foldable
Gas laws foldable Unit 6 review questions
Unit 6 review questions Joule si unit
Joule si unit Si unit for kinetic energy
Si unit for kinetic energy Chapter 14 solids liquids and gases
Chapter 14 solids liquids and gases The kinetic theory of matter states that
The kinetic theory of matter states that Kinetic theory of matter definition
Kinetic theory of matter definition Kinetic theory of matter
Kinetic theory of matter An explanation of how particles in matter behave
An explanation of how particles in matter behave Kinetic molecular theory of gases
Kinetic molecular theory of gases Kinetic molecular theory volume
Kinetic molecular theory volume Adhesive force
Adhesive force Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Kinetic molecular theory
Kinetic molecular theory Kinetic molecular theory def
Kinetic molecular theory def Particle theory evaporation
Particle theory evaporation Kinetic molecular theory timeline
Kinetic molecular theory timeline Kinetic theory of solids
Kinetic theory of solids Kinetic theory of gases
Kinetic theory of gases Charles law in terms of kinetic molecular theory
Charles law in terms of kinetic molecular theory Kmt law
Kmt law Kinetic theory of gases
Kinetic theory of gases What is a kinetic theory of matter
What is a kinetic theory of matter Postulates of kinetic theory of gases
Postulates of kinetic theory of gases