Unit 6 Kinetic Molecular Theory and Gas Laws




- Slides: 30

Unit 6: Kinetic Molecular Theory and Gas Laws CH 1120

Gases � Vary with respect to chemical properties but share some physical properties ◦ ◦ ◦ Low molar masses Non-metallic Expand spontaneously to fill container Volume of gas = volume of container Compressible Form homogeneous mixtures readily � All physical properties arise due to molecules being relatively far apart

Gases � At ◦ ◦ ◦ ◦ ◦ room temperature: He Ne Ar Kr Xe H 2 N 2 O 2 F 2 Cl 2

Kinetic Molecular Theory (Gases) � An increase in volume at a constant temperature causes pressure to decrease ◦ Syringe filled with air �A temperature increase at constant volume causes pressure to increase ◦ Balloon in winter

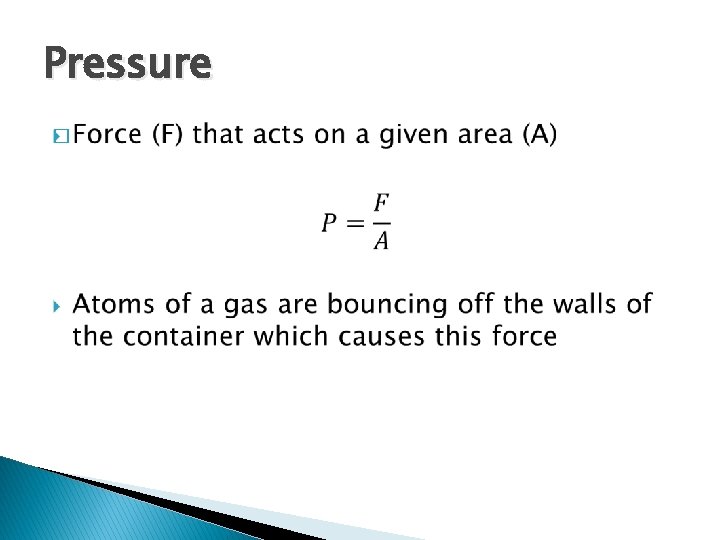
Pressure �

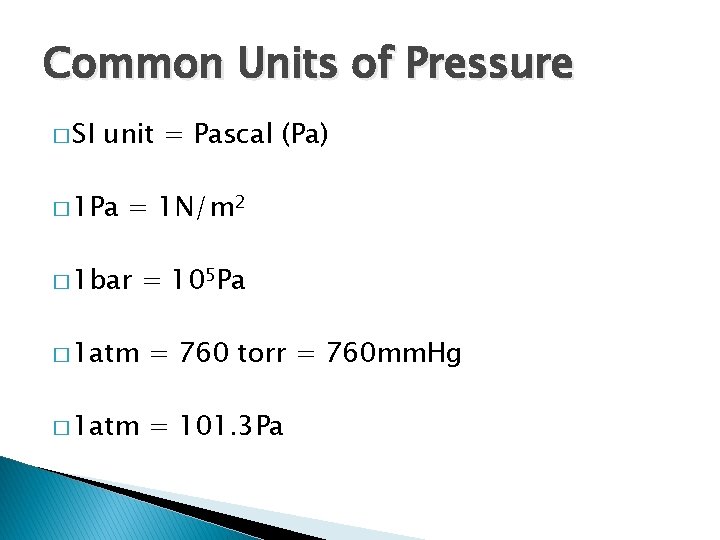
Common Units of Pressure � SI unit = Pascal (Pa) � 1 Pa = 1 N/m 2 � 1 bar = 105 Pa � 1 atm = 760 torr = 760 mm. Hg � 1 atm = 101. 3 Pa

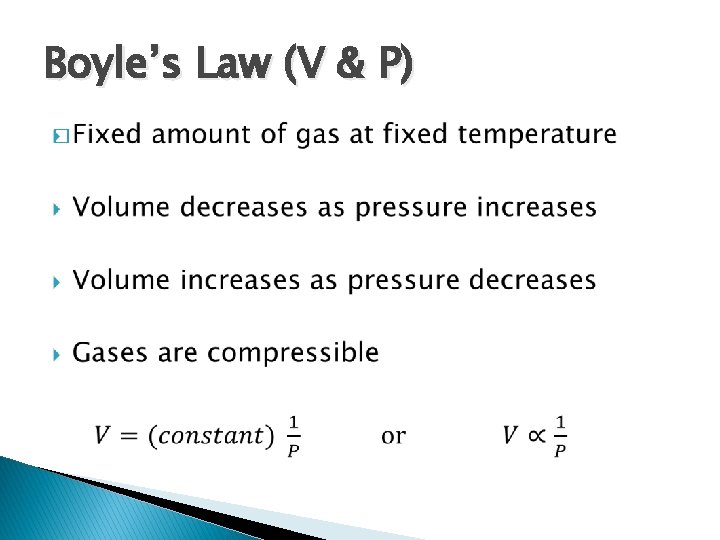
Boyle’s Law (V & P) �

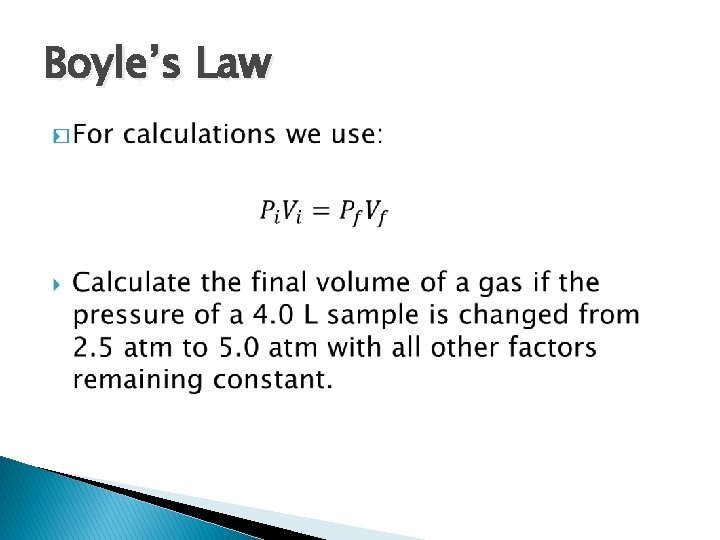
Boyle’s Law �

Kelvin � Remember �K how to calculate K = ℃ + 273. 15 � Used in gas law equations

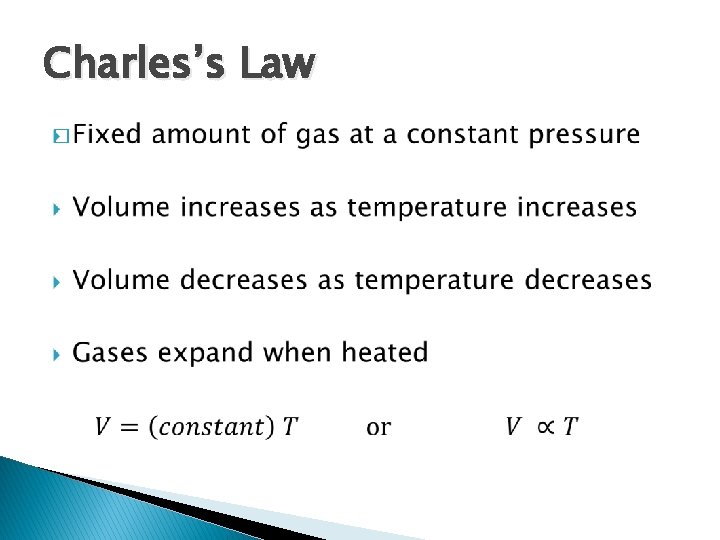
Charles’s Law �

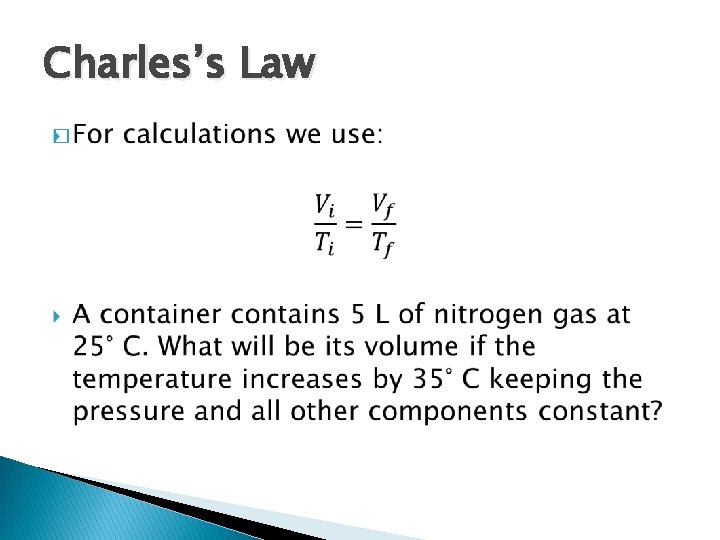
Charles’s Law �

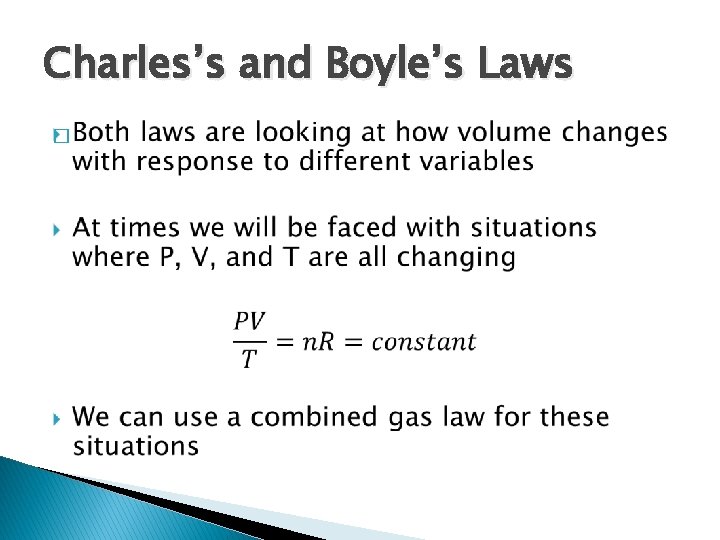
Charles’s and Boyle’s Laws �

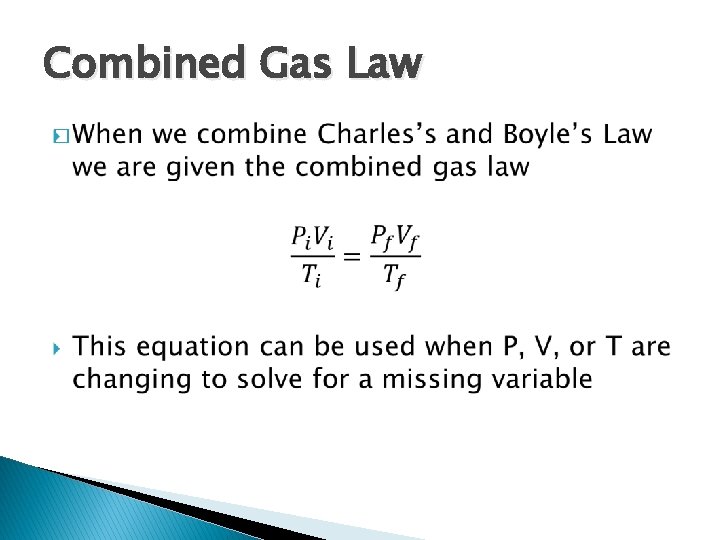
Combined Gas Law �

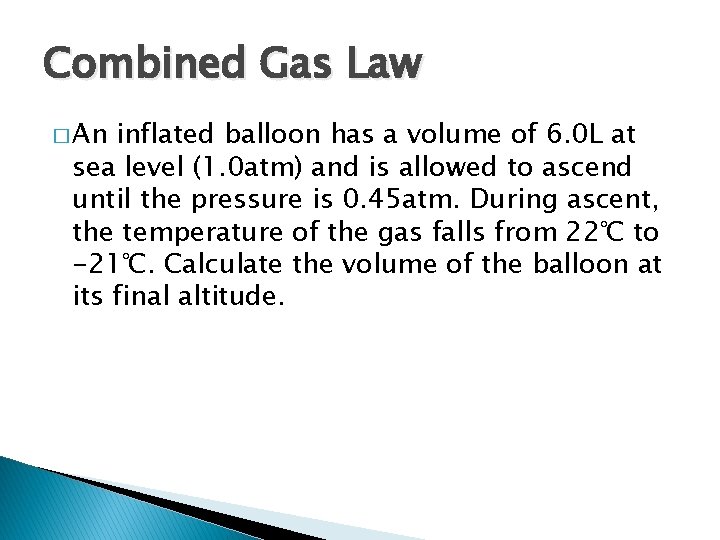
Combined Gas Law � An inflated balloon has a volume of 6. 0 L at sea level (1. 0 atm) and is allowed to ascend until the pressure is 0. 45 atm. During ascent, the temperature of the gas falls from 22℃ to -21℃. Calculate the volume of the balloon at its final altitude.

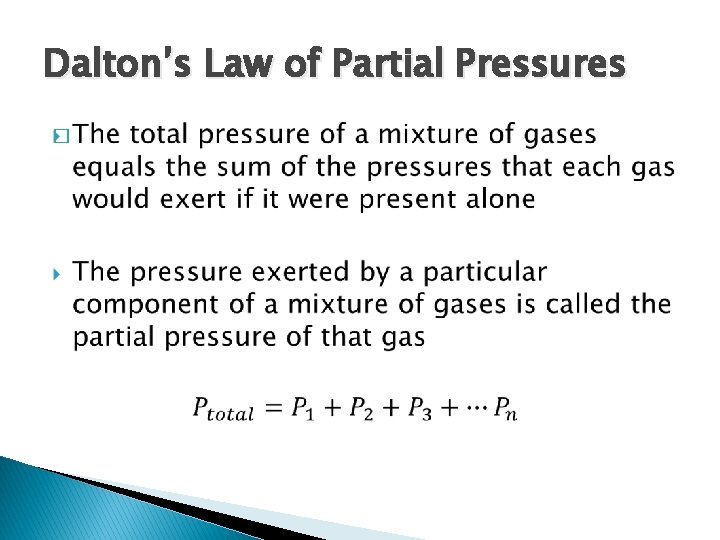
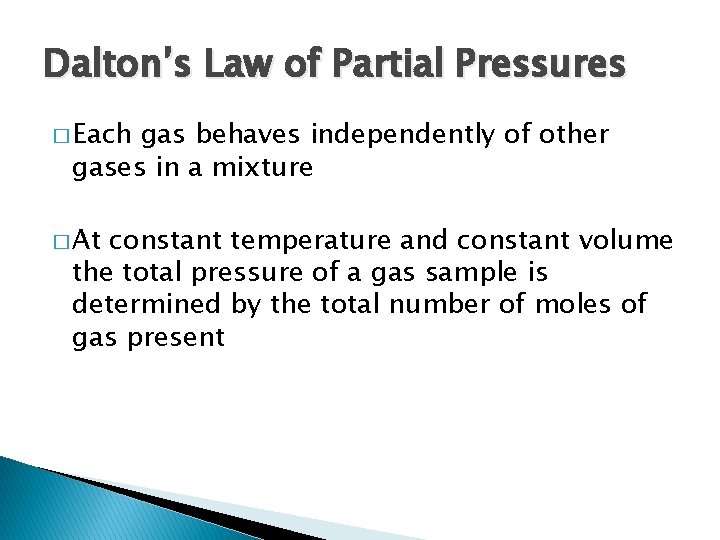
Dalton’s Law of Partial Pressures �

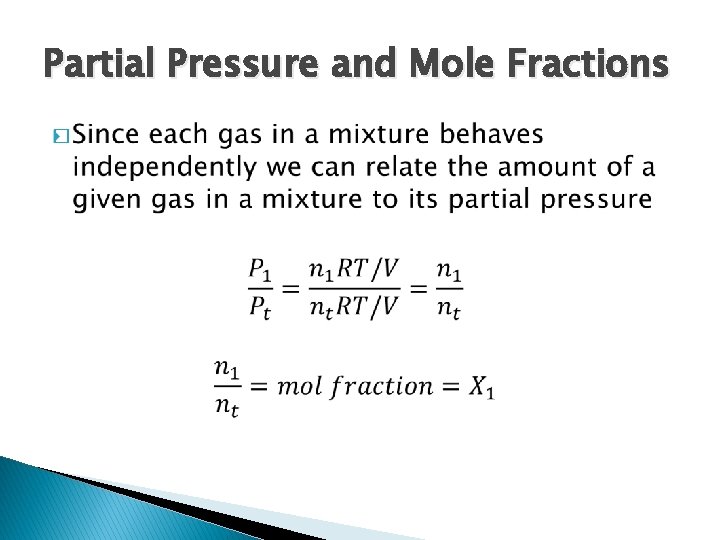
Dalton’s Law of Partial Pressures � Each gas behaves independently of other gases in a mixture � At constant temperature and constant volume the total pressure of a gas sample is determined by the total number of moles of gas present

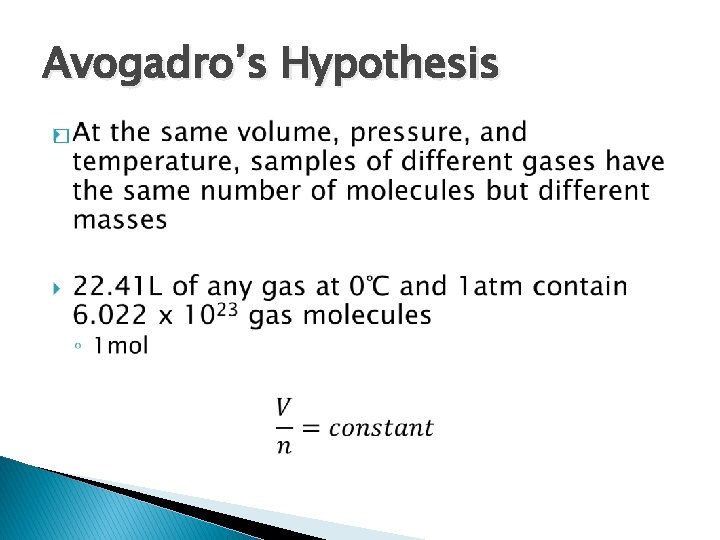
Avogadro’s Hypothesis �

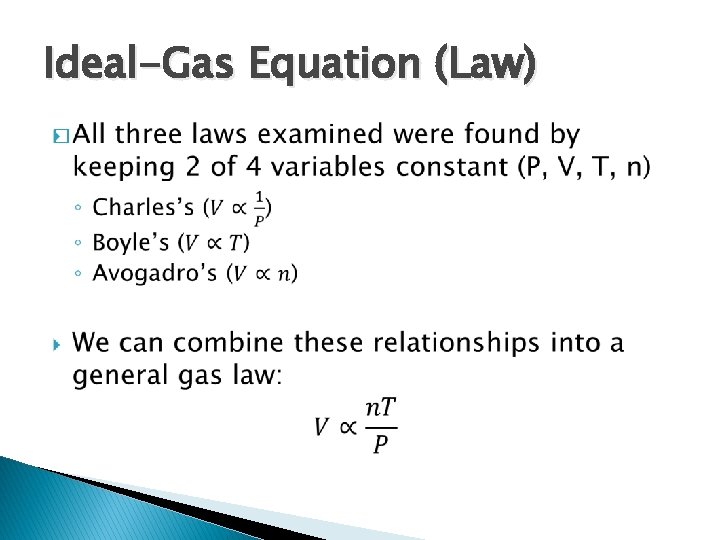
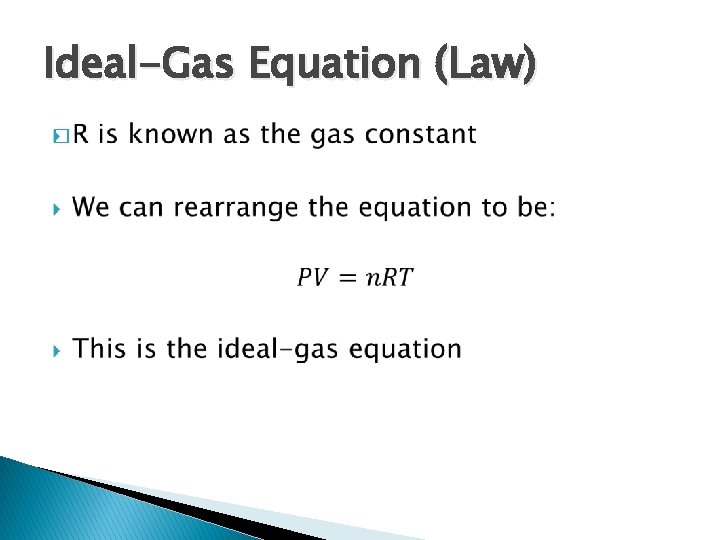
Ideal-Gas Equation (Law) �

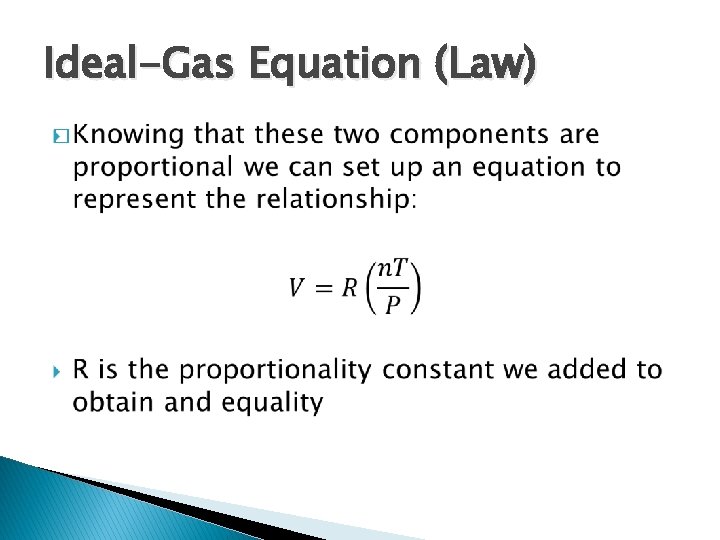
Ideal-Gas Equation (Law) �

Ideal-Gas Equation (Law) �

What is an Ideal Gas? � Hypothetical gas whose pressure, volume, and temperature relationship are described completely by the ideal gas equation ◦ The molecules do not interact with one another ◦ The volume of the molecules is much smaller than the volume the gas occupies so we assume the molecules take up no space in the container � Very small error is introduced with these assumptions so it is acceptable ◦ When accurate calculations are needed we can correct for the assumptions

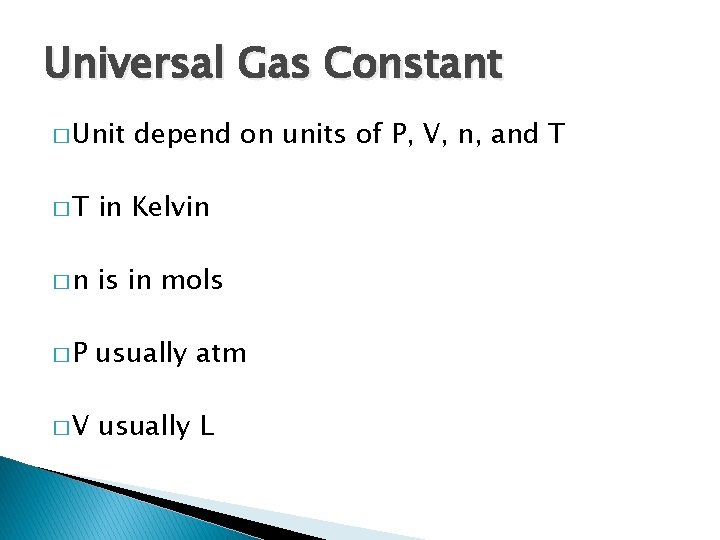
Universal Gas Constant � Unit depend on units of P, V, n, and T �T in Kelvin �n is in mols �P usually atm �V usually L

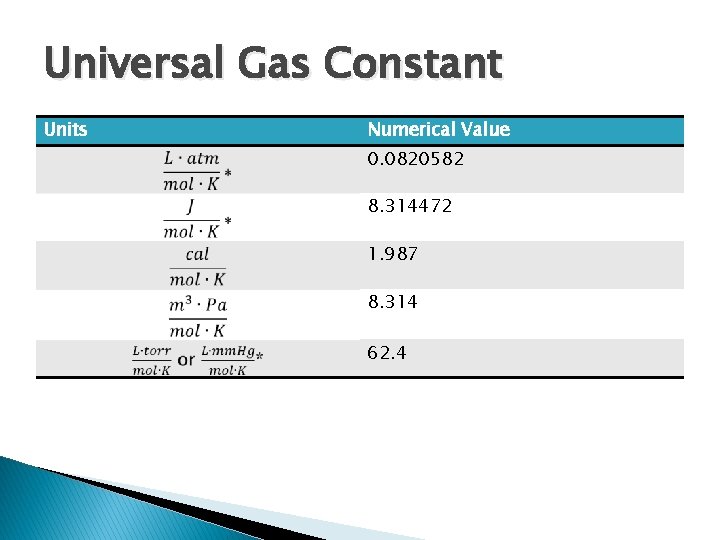
Universal Gas Constant Units Numerical Value 0. 0820582 8. 314472 1. 987 8. 314 62. 4

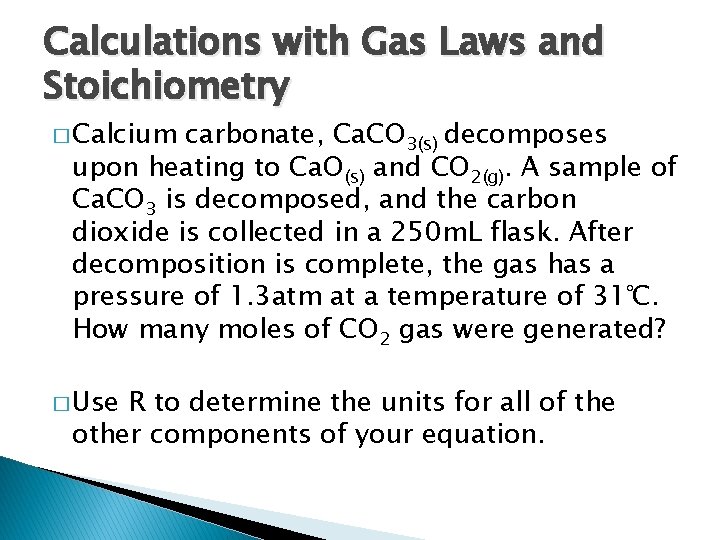
Calculations with Gas Laws and Stoichiometry � Calcium carbonate, Ca. CO 3(s) decomposes upon heating to Ca. O(s) and CO 2(g). A sample of Ca. CO 3 is decomposed, and the carbon dioxide is collected in a 250 m. L flask. After decomposition is complete, the gas has a pressure of 1. 3 atm at a temperature of 31℃. How many moles of CO 2 gas were generated? � Use R to determine the units for all of the other components of your equation.

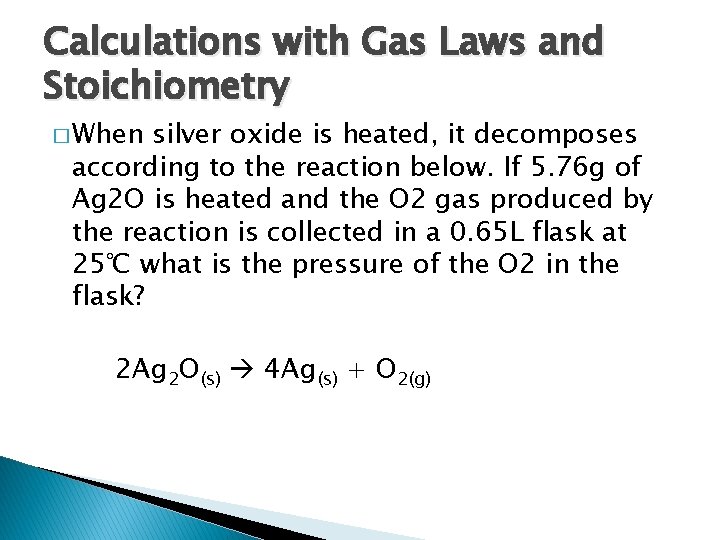
Calculations with Gas Laws and Stoichiometry � When silver oxide is heated, it decomposes according to the reaction below. If 5. 76 g of Ag 2 O is heated and the O 2 gas produced by the reaction is collected in a 0. 65 L flask at 25℃ what is the pressure of the O 2 in the flask? 2 Ag 2 O(s) 4 Ag(s) + O 2(g)

Partial Pressure and Mole Fractions �

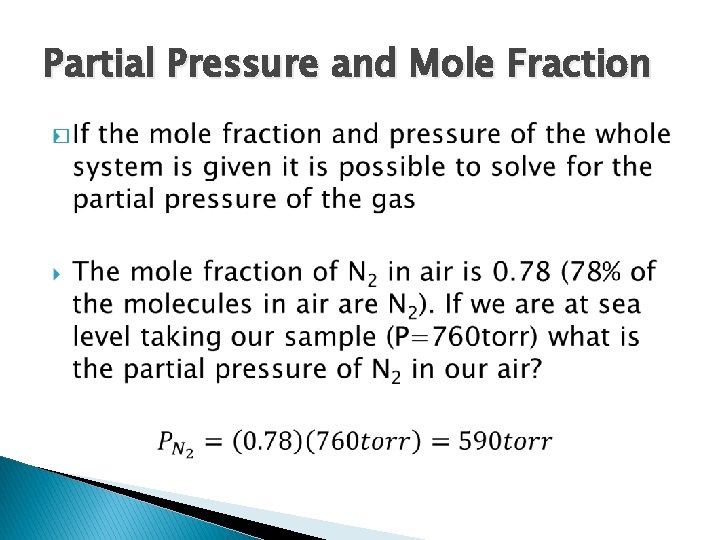
Partial Pressure and Mole Fraction �

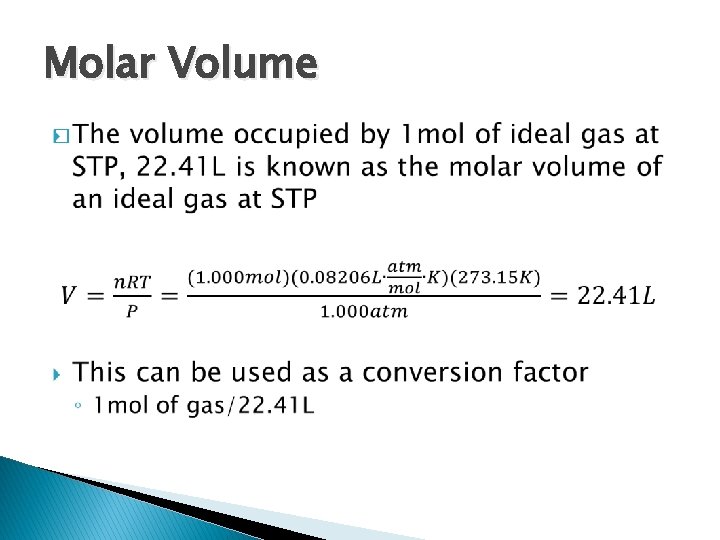
Standard Temperature and Pressure � 0℃ and 1 atm are referred to as STP ◦ Standard temperature and pressure

Molar Volume �

Molar Volume �A sample of pure helium gas occupies a volume of 6. 8 L at STP, how many grams of helium are present?
 Chapter 14 solids liquids and gases
Chapter 14 solids liquids and gases Adhesive force
Adhesive force Kinetic theory for ideal gases
Kinetic theory for ideal gases Kinetic molecular theory volume
Kinetic molecular theory volume Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Kinetic molecular theory
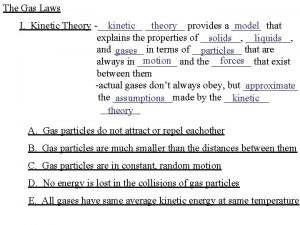
Kinetic molecular theory Kinetic molecular theory def
Kinetic molecular theory def Kinetic molecular theory timeline
Kinetic molecular theory timeline Charles law in terms of kinetic molecular theory
Charles law in terms of kinetic molecular theory Kinetic theory of gases postulates
Kinetic theory of gases postulates Avogadro's law
Avogadro's law Write postulates of kinetic theory of gases
Write postulates of kinetic theory of gases Kinetic molecular theory formula
Kinetic molecular theory formula Kinetic molecular theory
Kinetic molecular theory Tenets of kinetic molecular theory
Tenets of kinetic molecular theory Molecular orbital theory vs valence bond theory
Molecular orbital theory vs valence bond theory Slidetodoc.com
Slidetodoc.com Valence bond theory and molecular orbital theory
Valence bond theory and molecular orbital theory Valence bond theory and molecular orbital theory
Valence bond theory and molecular orbital theory Bond order of li2
Bond order of li2 Relation between pressure and kinetic energy of gas
Relation between pressure and kinetic energy of gas Covalently bonded substances
Covalently bonded substances Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Facts about montesquieu
Facts about montesquieu Indirect relationship example
Indirect relationship example Kinetic energy of gas formula
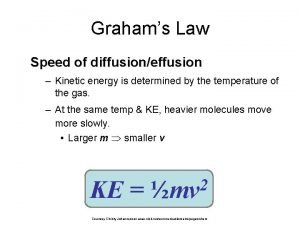
Kinetic energy of gas formula Graham's law of diffusion formula
Graham's law of diffusion formula Gas laws crash course
Gas laws crash course What are the empirical gas laws
What are the empirical gas laws How to find initial volume in boyle's law
How to find initial volume in boyle's law