Gas Laws The Kinetic Molecular Theory Gas particles





- Slides: 18

Gas Laws

The Kinetic Molecular Theory • Gas particles do not repel or attract each other. • Gas particles are much smaller than the distances between them. • Gas particles are in constant, random motion. • No kinetic energy is lost when gas particles collide. • All gases have the same kinetic energy at a given temperature.

Pressure • Pressure is force applied over an area. • For gases, the pressure is determined by the number of collisions with the walls of the container. • Units: – atm: atmospheres – k. Pa: kilo. Pascal (= 1 N/m 2) – mm. Hg: millimeters of mercury – psi: pound force per square inch

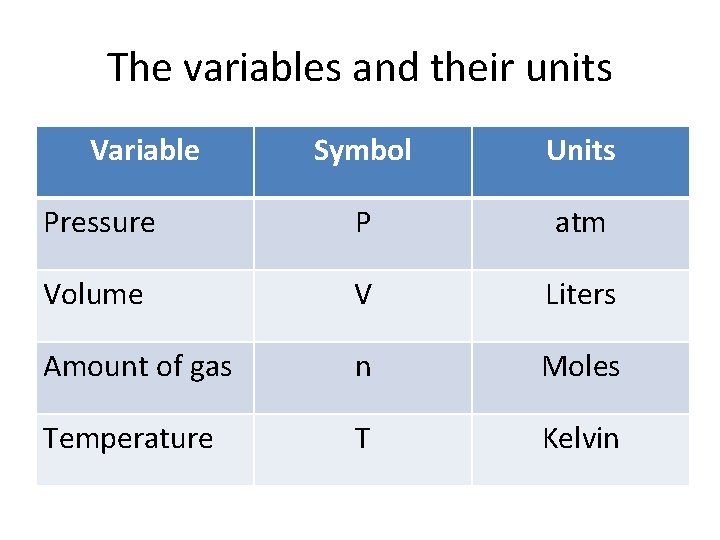
The variables and their units Variable Symbol Units Pressure P atm Volume V Liters Amount of gas n Moles Temperature T Kelvin

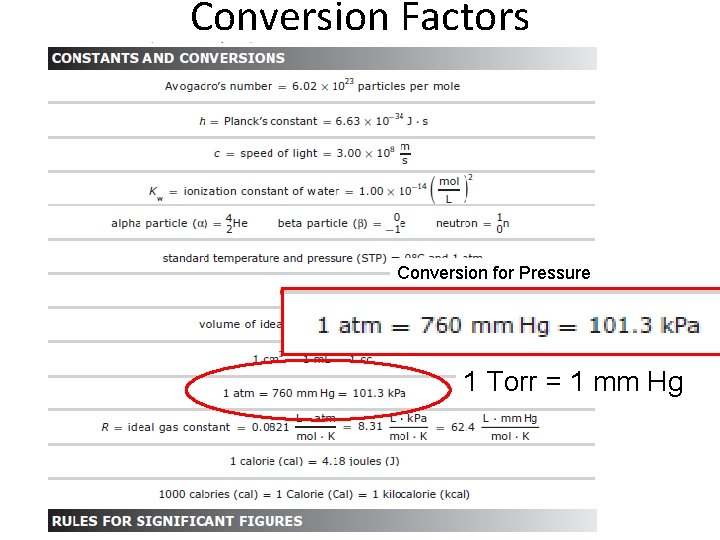
Conversion Factors Conversion for Pressure 1 Torr = 1 mm Hg

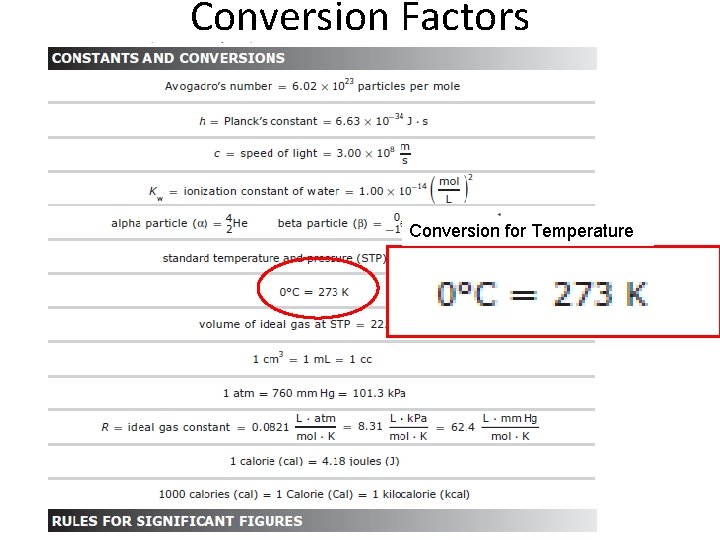
Conversion Factors Conversion for Temperature

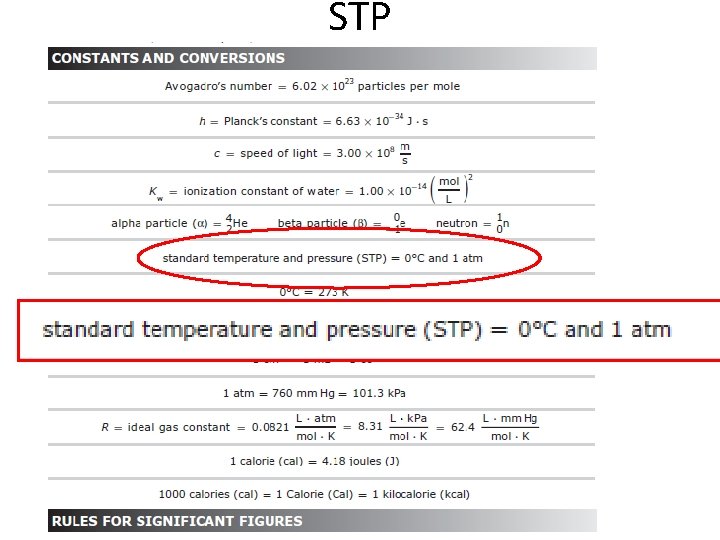
STP

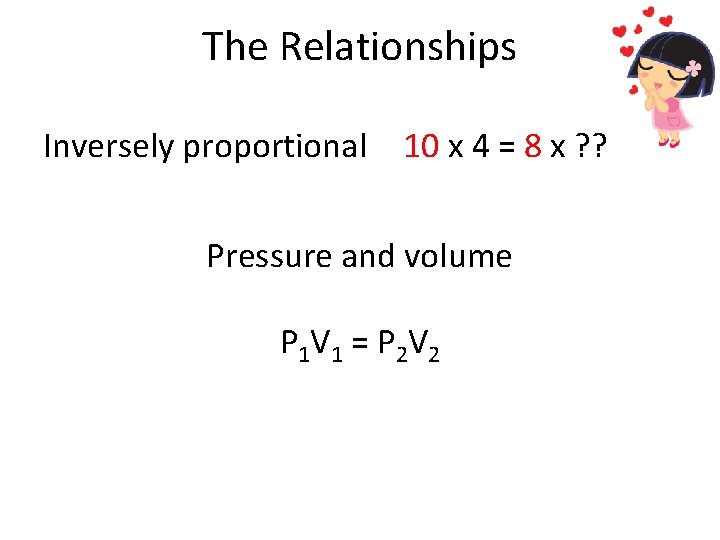
The Relationships before Copy in color: after 10 x 4 = 8 x ? ? • What is the missing number? • From before to after: – How did the red number change? – How did the black number change? • What is the math term for this relationship? • Which two gas variables have this type of relationship?

The Relationships Inversely proportional 10 x 4 = 8 x ? ? Pressure and volume P 1 V 1 = P 2 V 2

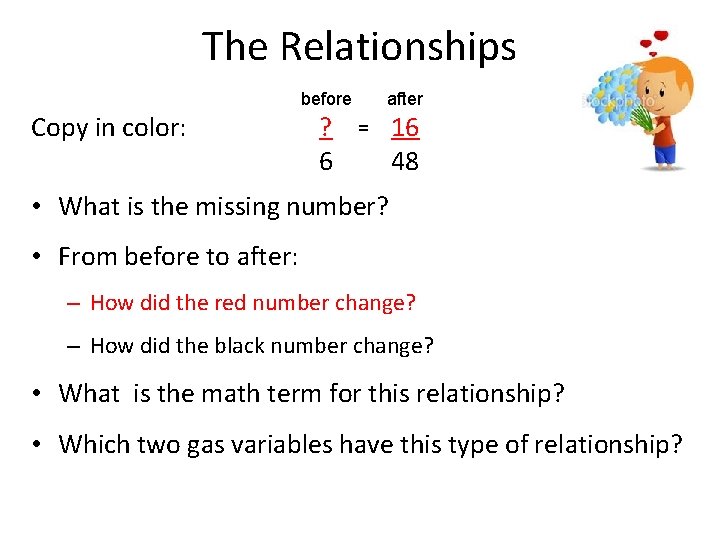
The Relationships before Copy in color: ? 6 after = 16 48 • What is the missing number? • From before to after: – How did the red number change? – How did the black number change? • What is the math term for this relationship? • Which two gas variables have this type of relationship?

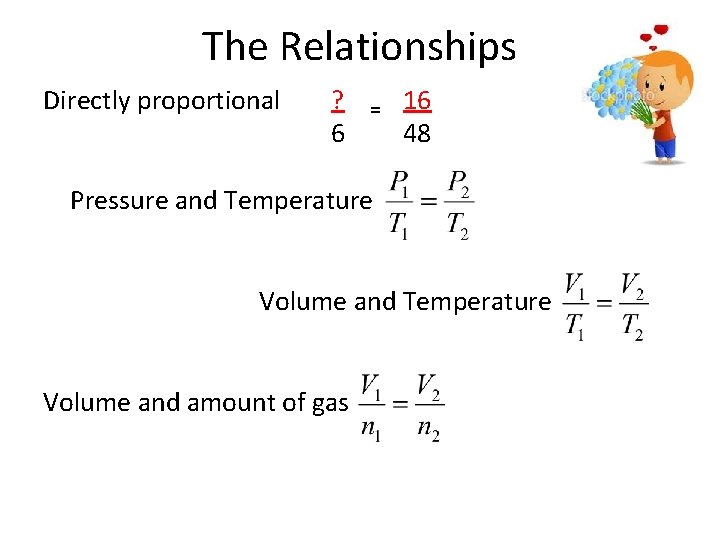
The Relationships Directly proportional ? 6 = 16 48 Pressure and Temperature Volume and amount of gas

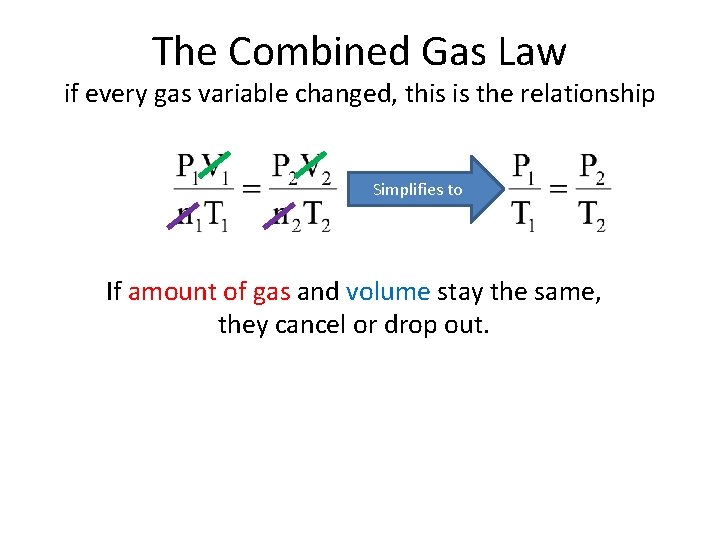
The Combined Gas Law if every gas variable changed, this is the relationship Things that don’t change cancel or drop out.

The Combined Gas Law if every gas variable changed, this is the relationship Simplifies to If amount of gas and volume stay the same, they cancel or drop out.

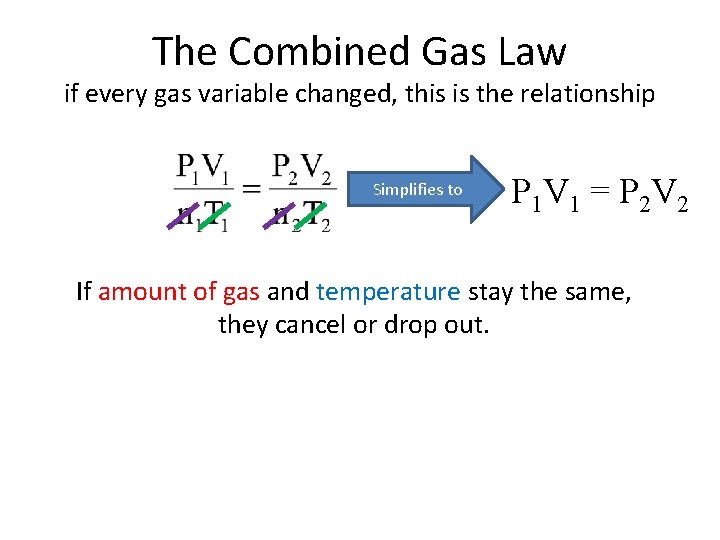
The Combined Gas Law if every gas variable changed, this is the relationship Simplifies to P 1 V 1 = P 2 V 2 If amount of gas and temperature stay the same, they cancel or drop out.

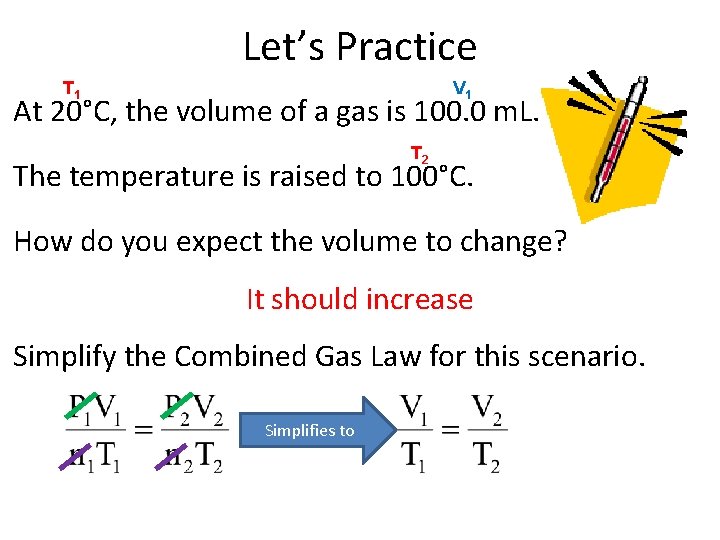
Let’s Practice T 1 V 1 At 20°C, the volume of a gas is 100. 0 m. L. T 2 The temperature is raised to 100°C. How do you expect the volume to change? It should increase Simplify the Combined Gas Law for this scenario. Simplifies to

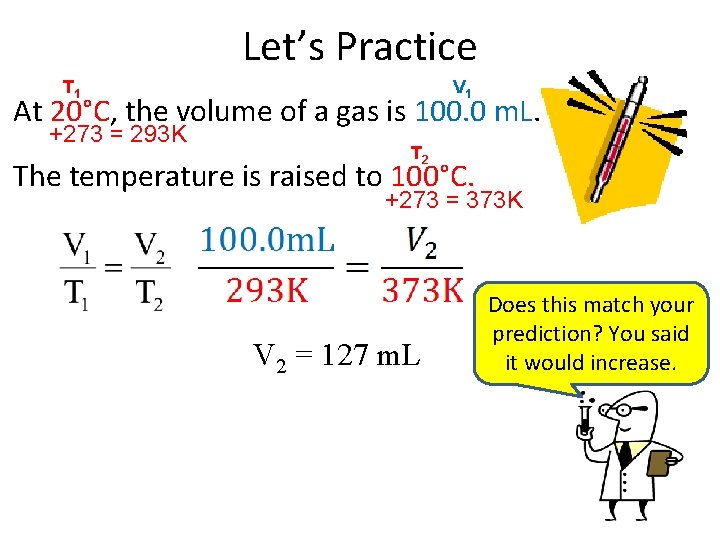
Let’s Practice T 1 V 1 At 20°C, the volume of a gas is 100. 0 m. L. +273 = 293 K T 2 The temperature is raised to 100°C. +273 = 373 K V 2 = 127 m. L Does this match your prediction? You said it would increase.

Let’s Practice V 1 P 1 50. 0 m. L of a gas has a pressure of 740. 0 mm. Hg when it is in a container. P 2 The pressure changes to 760. 0 mm. Hg. How do you expect the volume to change? It should decrease Simplify the Combined Gas Law for this scenario. Simplifies to P 1 V 1 = P 2 V 2

Let’s Practice V 1 P 1 50. 0 m. L of a gas has a pressure of 740. 0 mm. Hg when it is in a container. P 2 The pressure changes to 760. 0 mm. Hg? P 1 V 1 = P 2 V 2 (740. 0 mm. Hg)(50. 0 m. L) = (760. 0 mm. Hg)V 2 48. 7 m. L = V 2 Does this match your prediction? You said it would increase.
 Buoyancyability
Buoyancyability Chapter 14 solids liquids and gases
Chapter 14 solids liquids and gases Kinetic molecular theory
Kinetic molecular theory The kinetic molecular theory
The kinetic molecular theory Adhesive force
Adhesive force Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Postulates of kinetic molecular theory
Postulates of kinetic molecular theory Kinetic molecular theory def
Kinetic molecular theory def Kinetic molecular theory timeline
Kinetic molecular theory timeline Charles law in terms of kinetic molecular theory
Charles law in terms of kinetic molecular theory Postulates of kinetic theory
Postulates of kinetic theory Kmt law
Kmt law Postulates of kinetic theory of gases
Postulates of kinetic theory of gases Kinetic molecular theory formula
Kinetic molecular theory formula Kinetic molecular theory
Kinetic molecular theory Tenets of kinetic molecular theory
Tenets of kinetic molecular theory Comparison between mot and vbt
Comparison between mot and vbt Dxz and dxz overlap
Dxz and dxz overlap Valence bond theory and molecular orbital theory
Valence bond theory and molecular orbital theory