Gas Laws Kinetic Molecular Theory The average kinetic












- Slides: 26

Gas Laws

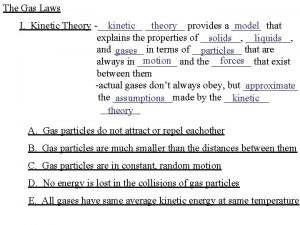
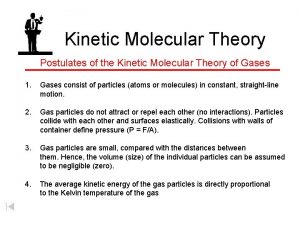
Kinetic Molecular Theory The average kinetic energy (energy of motion ) is directly proportional to absolute temperature (Kelvin temperature) of a gas Example Average energy determines the temperature of the sample ; If absolute temperature doubles, the average kinetic energy doubles Kinetic energy increases , particles move faster , number of collisions increase (explains why gases expand when heated)

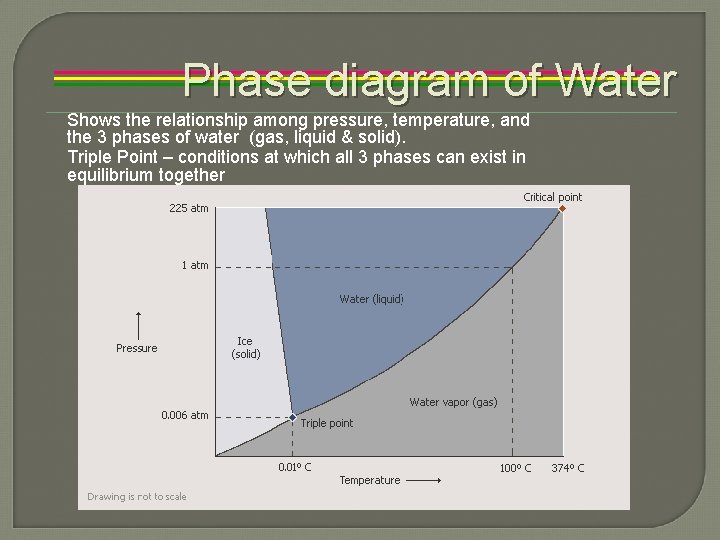
Phase diagram of Water Shows the relationship among pressure, temperature, and the 3 phases of water (gas, liquid & solid). Triple Point – conditions at which all 3 phases can exist in equilibrium together

Definitions Heat • Energy transferred due to differences in temperature Temperature • Measure of the average kinetic energy of particles composing a material Pressure • Force per unit area Volume • The amount of space a material occupies

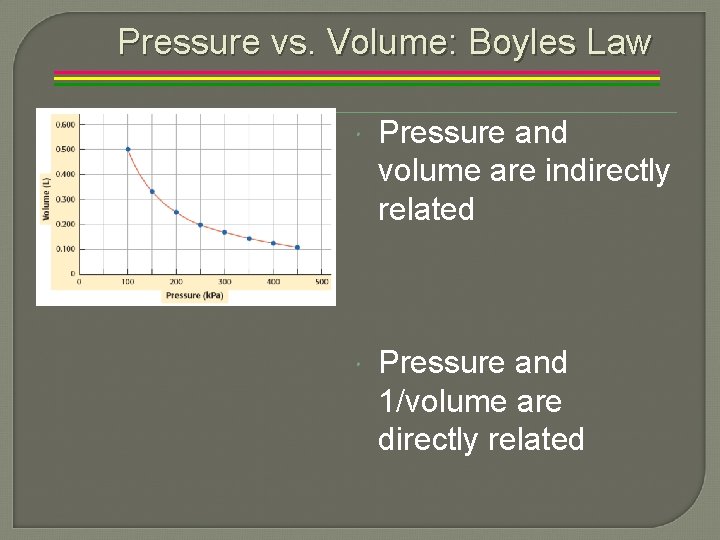
Pressure vs. Volume: Boyles Law Pressure and volume are indirectly related Pressure and 1/volume are directly related

Gas Law Problems: Boyles’ Law 1. 2. A balloon initially occupies 12. 4 L at 1. 00 atm. What will be the volume at 0. 800 atm? A sample of gas expands from 10. 0 L to 30. 0 L. If the initial pressure was 1140 mm Hg, what is the new pressure?

Using Boyles law Calculate: A balloon contains 40. 0 L of a gas at 101. 3 k. Pa. What is the volume of the gas when the balloon is brought to an altitude where the pressure is only 10. 0 k. Pa? (Assume that the temperature remains constant. )

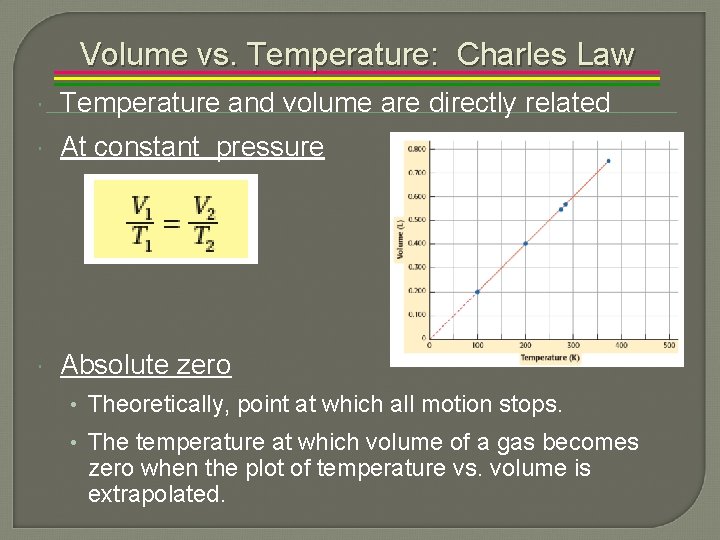
Volume vs. Temperature: Charles Law Temperature and volume are directly related At constant pressure Absolute zero • Theoretically, point at which all motion stops. • The temperature at which volume of a gas becomes zero when the plot of temperature vs. volume is extrapolated.

Gas Law Problems: Charles’Law 1. Calculate the decrease in temperature when 2. 00 L at 20. 0°C is compressed to 1. 00 L. 2. A gas occupies 900. 0 m. L at a temperature of 27. 0°C. What is the volume at 132. 0°C?

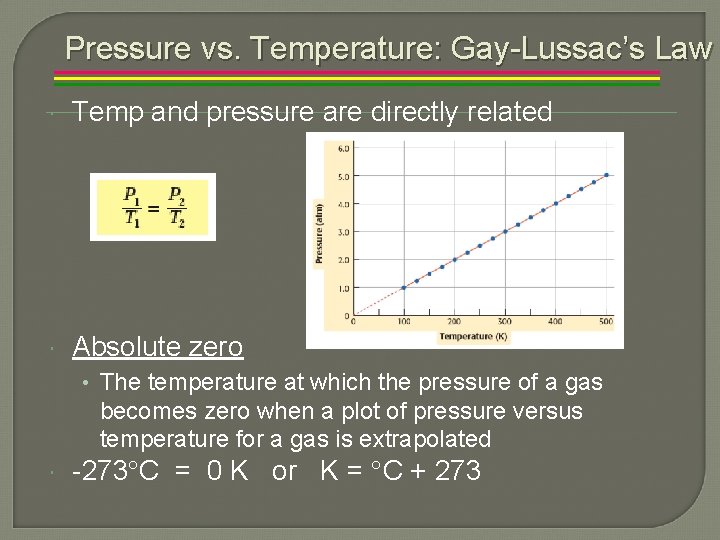
Pressure vs. Temperature: Gay-Lussac’s Law Temp and pressure are directly related Absolute zero • The temperature at which the pressure of a gas becomes zero when a plot of pressure versus temperature for a gas is extrapolated -273 C = 0 K or K = C + 273

Gas Law Problems: Gay-Lussac’s 1. Consider a container with a volume of 22. 4 L filled with a gas at 1. 00 atm at 273 K. What will be the new pressure if the temperature increases to 298 K?

Gas Law Problems: Gay-Lussac’s 2. A container is initially at 47 mm Hg and 77 K (liquid nitrogen temperature. ) What will the pressure be when the container warms up to room temperature of 25 C?

Gas Law Problems: Gay-Lussac’s 3. A thermometer reads a pressure of 248 Torr at 0. 0 C. What is the temperature when thermometer reads a pressure of 345 Torr?

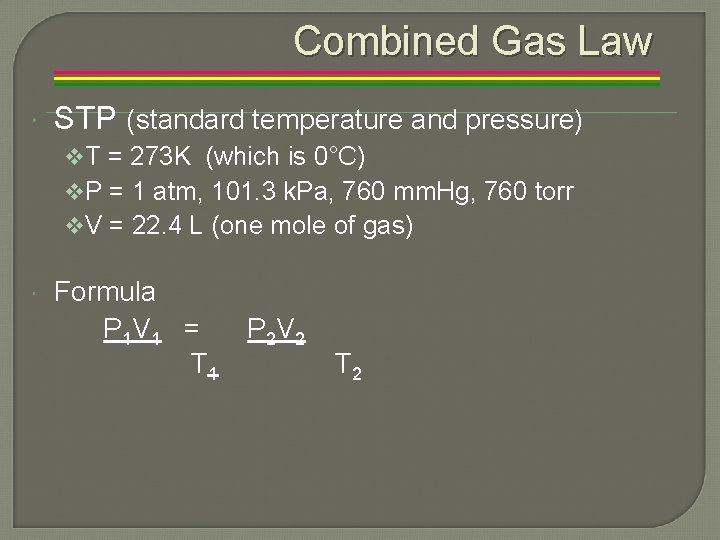
Combined Gas Law STP (standard temperature and pressure) v. T = 273 K (which is 0°C) v. P = 1 atm, 101. 3 k. Pa, 760 mm. Hg, 760 torr v. V = 22. 4 L (one mole of gas) Formula P 1 V 1 = T 1 P 2 V 2 T 2

Combined Gas Law 10. 0 cm 3 volume of a gas measured 75. 6 k. Pa and 60. 0 C is to be corrected to correspond to the volume it would occupy at STP.

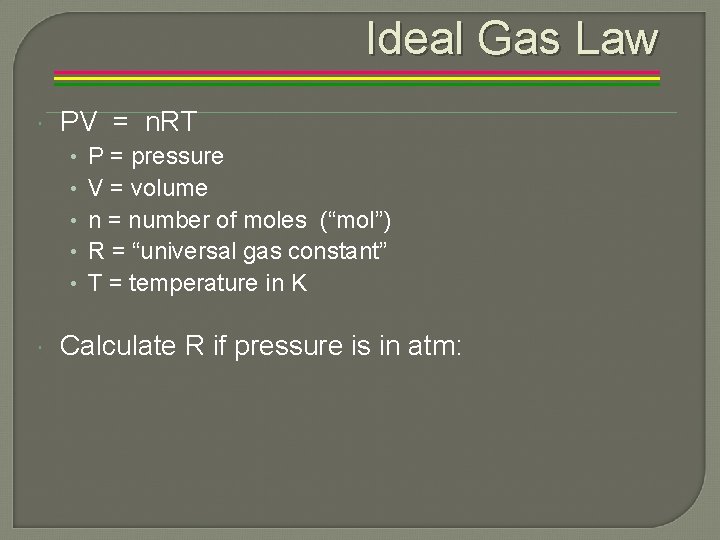
Ideal Gas Law PV = n. RT • • • P = pressure V = volume n = number of moles (“mol”) R = “universal gas constant” T = temperature in K Calculate R if pressure is in atm:

Ideal Gas Law Calculate R if pressure is in k. Pa: Calculate R if pressure is in torr or mm Hg:

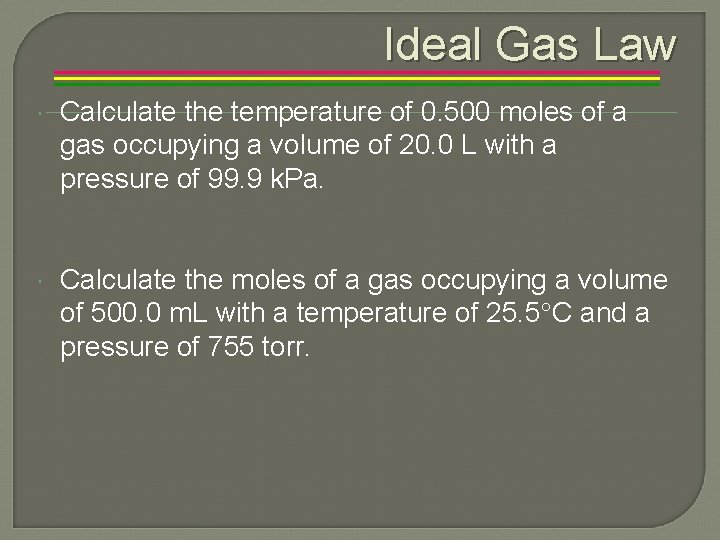
Ideal Gas Law Calculate the temperature of 0. 500 moles of a gas occupying a volume of 20. 0 L with a pressure of 99. 9 k. Pa. Calculate the moles of a gas occupying a volume of 500. 0 m. L with a temperature of 25. 5 C and a pressure of 755 torr.

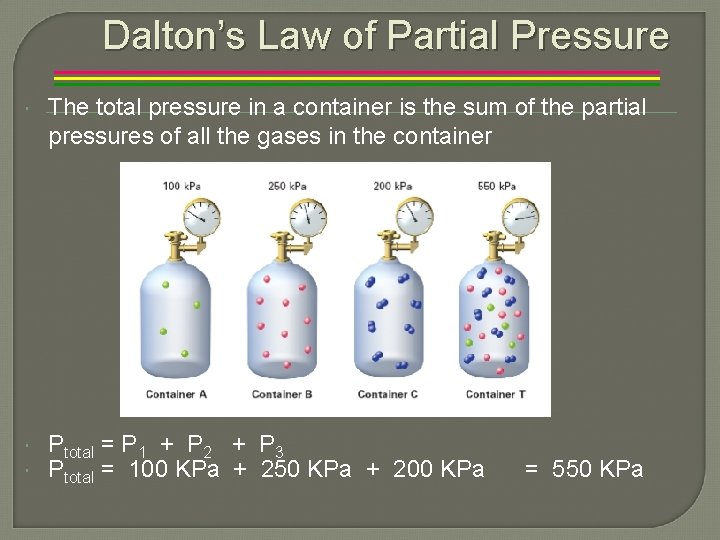
Dalton’s Law of Partial Pressure The total pressure in a container is the sum of the partial pressures of all the gases in the container Ptotal = P 1 + P 2 + P 3 Ptotal = 100 KPa + 250 KPa + 200 KPa = 550 KPa

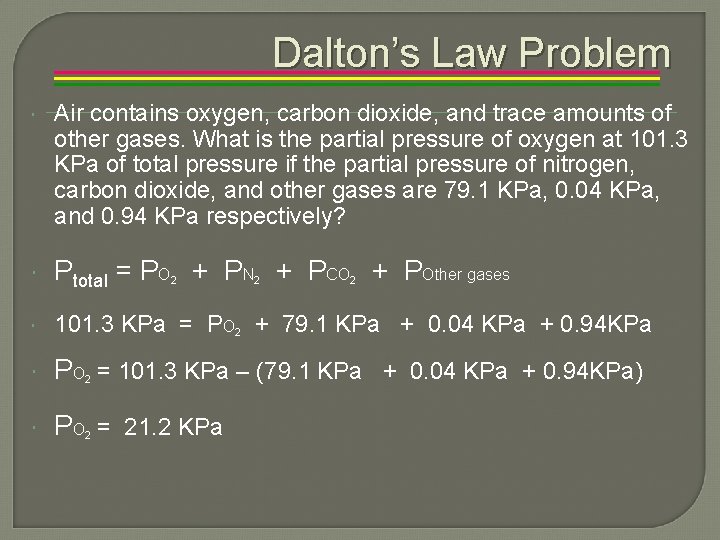
Dalton’s Law Problem Air contains oxygen, carbon dioxide, and trace amounts of other gases. What is the partial pressure of oxygen at 101. 3 KPa of total pressure if the partial pressure of nitrogen, carbon dioxide, and other gases are 79. 1 KPa, 0. 04 KPa, and 0. 94 KPa respectively? Ptotal = PO + PN + PCO + POther gases 101. 3 KPa = PO 2 + 79. 1 KPa + 0. 04 KPa + 0. 94 KPa PO = 101. 3 KPa – (79. 1 KPa + 0. 04 KPa + 0. 94 KPa) PO = 21. 2 KPa 2 2 2

Dalton’s Law Problem If a gas contains. 4 moles oxygen, . 3 moles of nitrogen, . 2 moles of hydrogen, and. 1 moles of argon, what is the partial pressure due to nitrogen?

Dalton’s Law Eudiometer Lab • Write the chemical equation that occurs when magnesium reacts with hydrochloric acid • Will collect the gas that goes through the water • Gas collected = H 2 + H 2 O vapor • To find the pressure of the H 2, we need to subtract the pressure water vapor

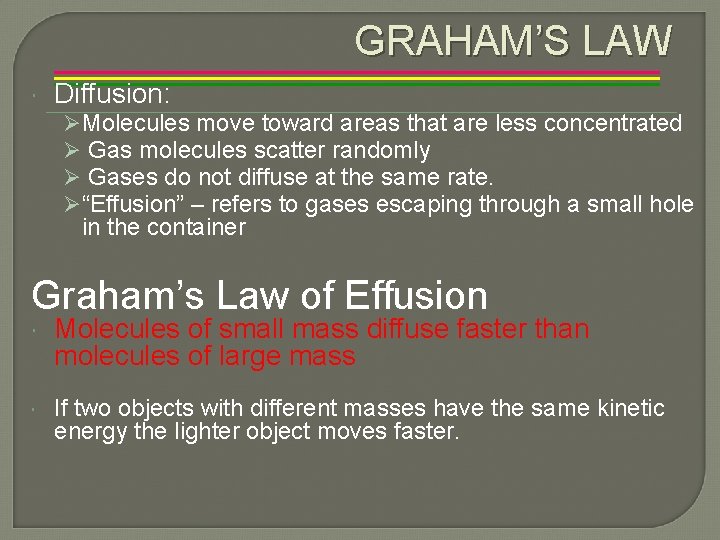
GRAHAM’S LAW Diffusion: ØMolecules move toward areas that are less concentrated Ø Gas molecules scatter randomly Ø Gases do not diffuse at the same rate. Ø“Effusion” – refers to gases escaping through a small hole in the container Graham’s Law of Effusion Molecules of small mass diffuse faster than molecules of large mass If two objects with different masses have the same kinetic energy the lighter object moves faster.

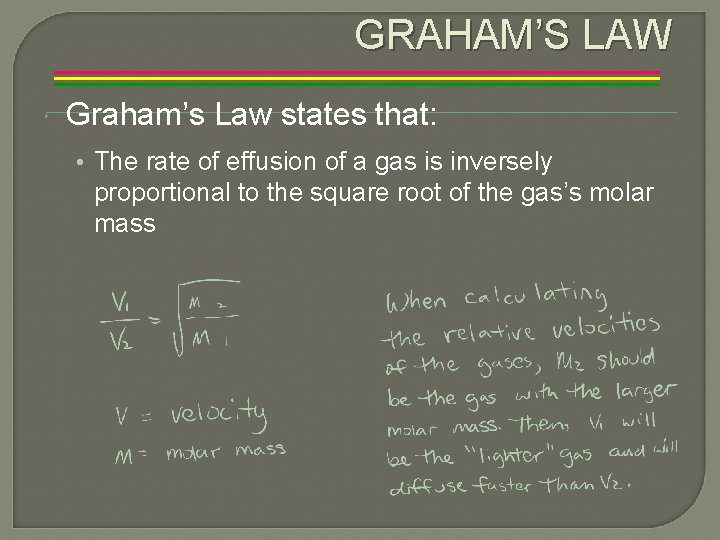
GRAHAM’S LAW Graham’s Law states that: • The rate of effusion of a gas is inversely proportional to the square root of the gas’s molar mass

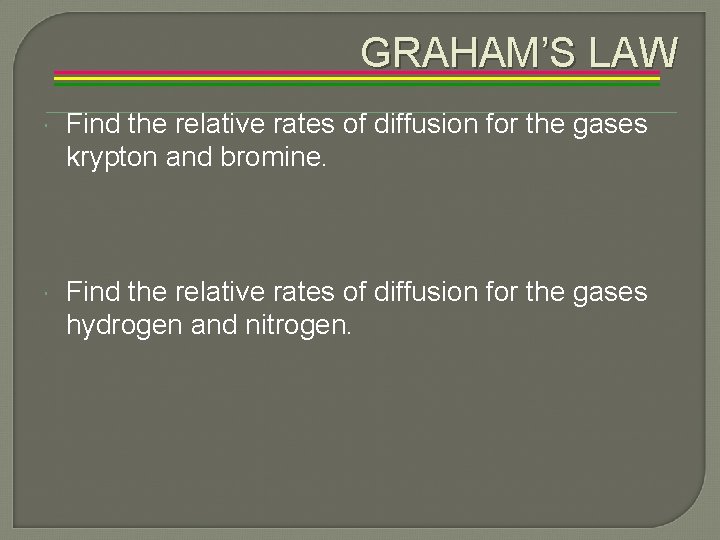
GRAHAM’S LAW Find the relative rates of diffusion for the gases krypton and bromine. Find the relative rates of diffusion for the gases hydrogen and nitrogen.

GRAHAM’S LAW Hydrogen gas diffuses 3. 724 times faster than gas A. What is the molar mass for gas A and what is gas A?
 Chapter 14 solids liquids and gases
Chapter 14 solids liquids and gases Kinetic molecular theory of gases
Kinetic molecular theory of gases The kinetic molecular theory
The kinetic molecular theory Adhesive force
Adhesive force Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Kinetic energy molecular theory
Kinetic energy molecular theory Kinetic theory def
Kinetic theory def Kinetic molecular theory timeline
Kinetic molecular theory timeline Charles law in terms of kinetic molecular theory
Charles law in terms of kinetic molecular theory Postulates of kinetic theory of gas
Postulates of kinetic theory of gas Kmt law
Kmt law Kinetic molecular theory
Kinetic molecular theory Pv=1/3nmc^2
Pv=1/3nmc^2 Kinetic molecular theory
Kinetic molecular theory Tenets of kinetic molecular theory
Tenets of kinetic molecular theory Molecular orbital diagram of f2
Molecular orbital diagram of f2 Valence bond theory and molecular orbital theory difference
Valence bond theory and molecular orbital theory difference Valence bond theory and molecular orbital theory
Valence bond theory and molecular orbital theory 11:55
11:55 Valence bond theory example problems
Valence bond theory example problems Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Ionic covalent metallic
Ionic covalent metallic Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Polydispersity
Polydispersity Number-average molecular weight
Number-average molecular weight Facts about montesquieu
Facts about montesquieu Straight line motion
Straight line motion