CHEMISTRY 2 BONDING AND REACTIONS RATES OF REACTION

CHEMISTRY 2 BONDING AND REACTIONS & RATES OF REACTION & USING IONS IN SOLUTION
BONDING & REACTIONS
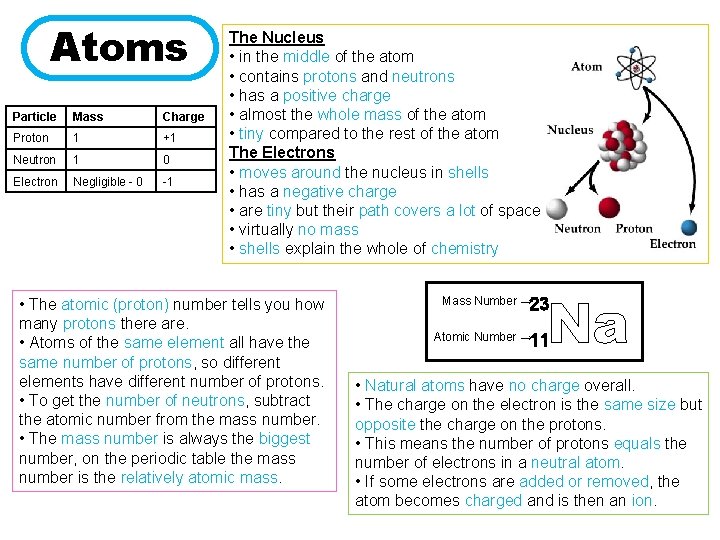
Atoms Particle Mass Charge Proton 1 +1 Neutron 1 0 Electron Negligible - 0 -1 The Nucleus • in the middle of the atom • contains protons and neutrons • has a positive charge • almost the whole mass of the atom • tiny compared to the rest of the atom The Electrons • moves around the nucleus in shells • has a negative charge • are tiny but their path covers a lot of space • virtually no mass • shells explain the whole of chemistry • The atomic (proton) number tells you how many protons there are. • Atoms of the same element all have the same number of protons, so different elements have different number of protons. • To get the number of neutrons, subtract the atomic number from the mass number. • The mass number is always the biggest number, on the periodic table the mass number is the relatively atomic mass. Na Mass Number → 23 Atomic Number → 11 • Natural atoms have no charge overall. • The charge on the electron is the same size but opposite the charge on the protons. • This means the number of protons equals the number of electrons in a neutral atom. • If some electrons are added or removed, the atom becomes charged and is then an ion.
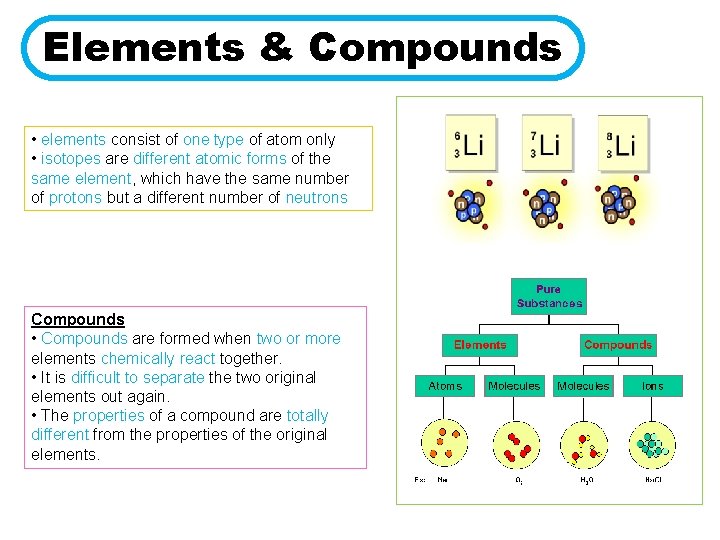
Elements & Compounds • elements consist of one type of atom only • isotopes are different atomic forms of the same element, which have the same number of protons but a different number of neutrons Compounds • Compounds are formed when two or more elements chemically react together. • It is difficult to separate the two original elements out again. • The properties of a compound are totally different from the properties of the original elements.
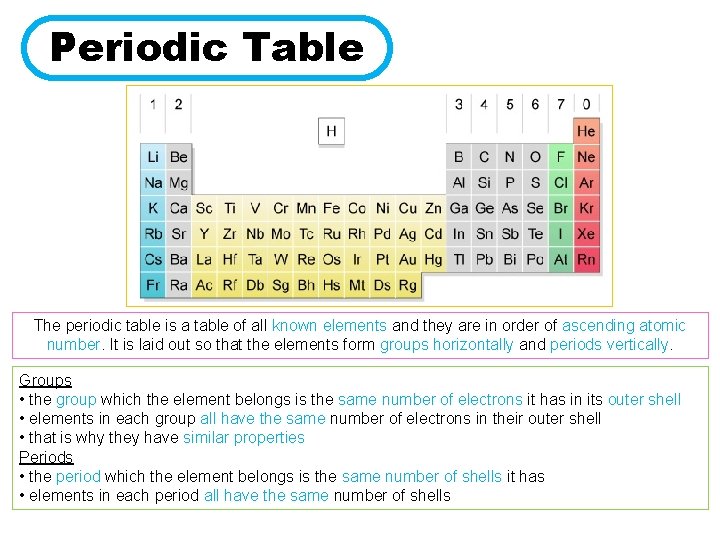
Periodic Table The periodic table is a table of all known elements and they are in order of ascending atomic number. It is laid out so that the elements form groups horizontally and periods vertically. Groups • the group which the element belongs is the same number of electrons it has in its outer shell • elements in each group all have the same number of electrons in their outer shell • that is why they have similar properties Periods • the period which the element belongs is the same number of shells it has • elements in each period all have the same number of shells
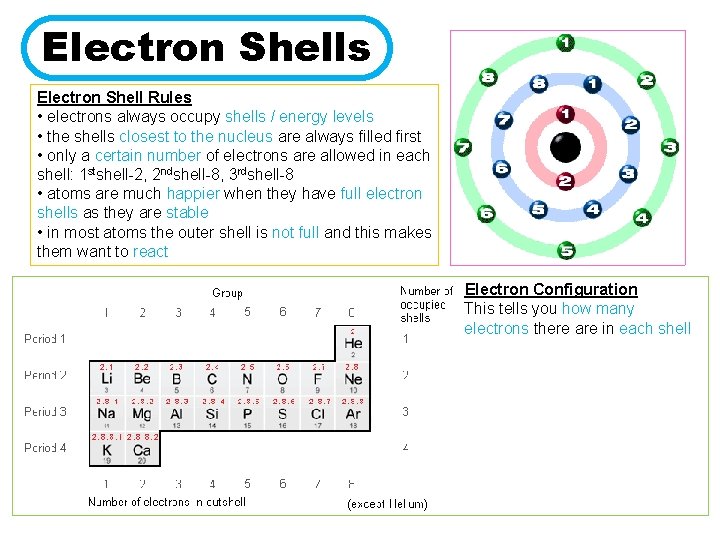
Electron Shells Electron Shell Rules • electrons always occupy shells / energy levels • the shells closest to the nucleus are always filled first • only a certain number of electrons are allowed in each shell: 1 stshell-2, 2 ndshell-8, 3 rdshell-8 • atoms are much happier when they have full electron shells as they are stable • in most atoms the outer shell is not full and this makes them want to react Electron Configuration This tells you how many electrons there are in each shell
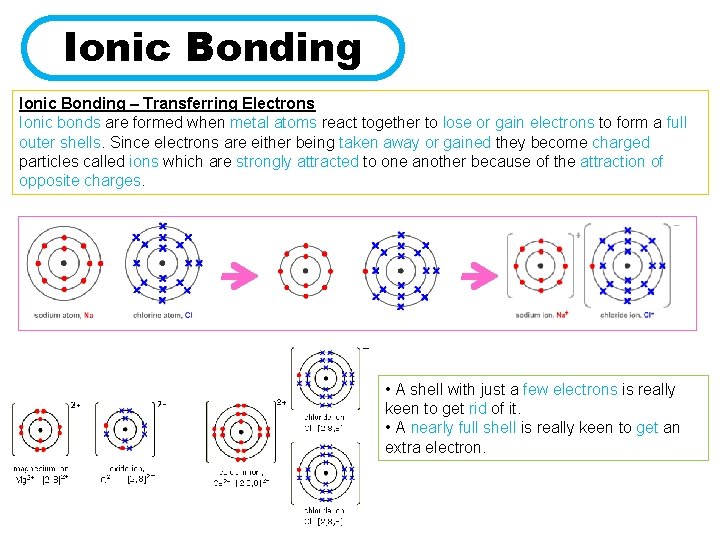
Ionic Bonding – Transferring Electrons Ionic bonds are formed when metal atoms react together to lose or gain electrons to form a full outer shells. Since electrons are either being taken away or gained they become charged particles called ions which are strongly attracted to one another because of the attraction of opposite charges. • A shell with just a few electrons is really keen to get rid of it. • A nearly full shell is really keen to get an extra electron.
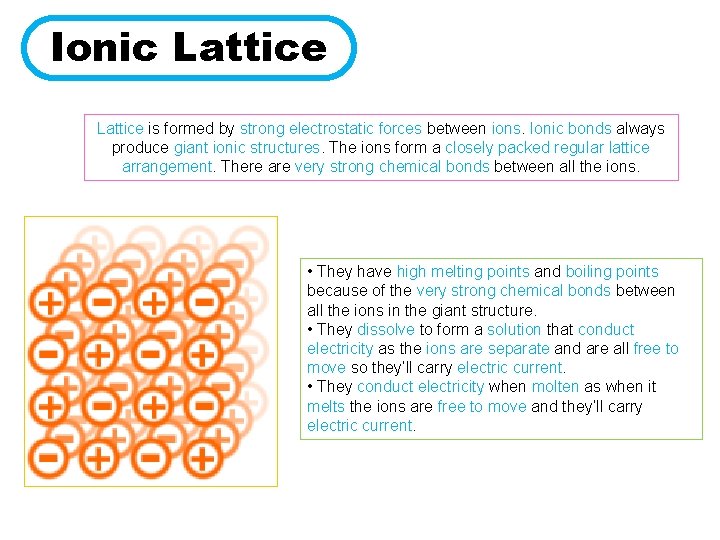
Ionic Lattice is formed by strong electrostatic forces between ions. Ionic bonds always produce giant ionic structures. The ions form a closely packed regular lattice arrangement. There are very strong chemical bonds between all the ions. • They have high melting points and boiling points because of the very strong chemical bonds between all the ions in the giant structure. • They dissolve to form a solution that conduct electricity as the ions are separate and are all free to move so they’ll carry electric current. • They conduct electricity when molten as when it melts the ions are free to move and they’ll carry electric current.
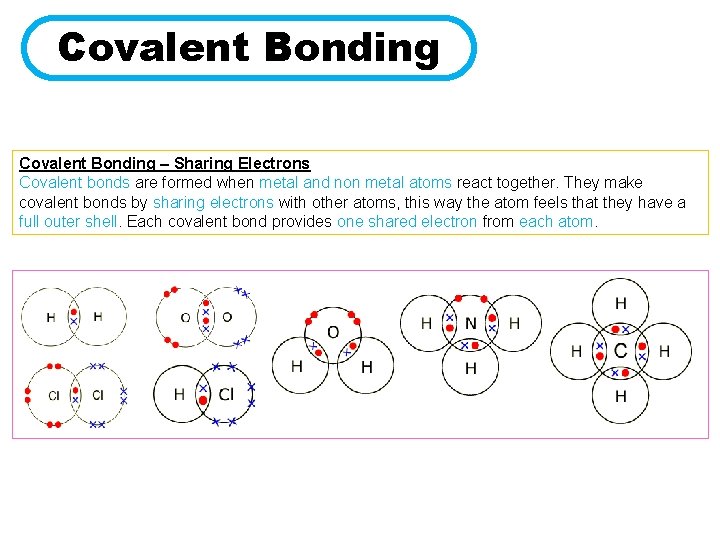
Covalent Bonding – Sharing Electrons Covalent bonds are formed when metal and non metal atoms react together. They make covalent bonds by sharing electrons with other atoms, this way the atom feels that they have a full outer shell. Each covalent bond provides one shared electron from each atom.
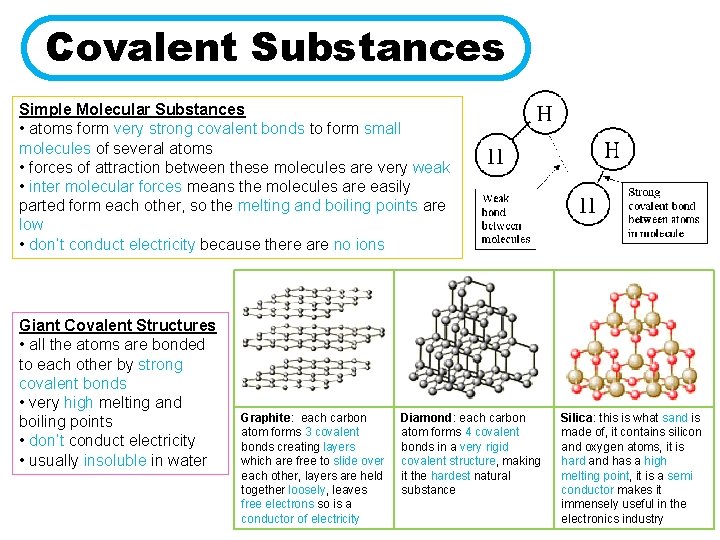
Covalent Substances Simple Molecular Substances • atoms form very strong covalent bonds to form small molecules of several atoms • forces of attraction between these molecules are very weak • inter molecular forces means the molecules are easily parted form each other, so the melting and boiling points are low • don’t conduct electricity because there are no ions Giant Covalent Structures • all the atoms are bonded to each other by strong covalent bonds • very high melting and boiling points • don’t conduct electricity • usually insoluble in water Graphite: each carbon atom forms 3 covalent bonds creating layers which are free to slide over each other, layers are held together loosely, leaves free electrons so is a conductor of electricity Diamond: each carbon atom forms 4 covalent bonds in a very rigid covalent structure, making it the hardest natural substance Silica: this is what sand is made of, it contains silicon and oxygen atoms, it is hard and has a high melting point, it is a semi conductor makes it immensely useful in the electronics industry
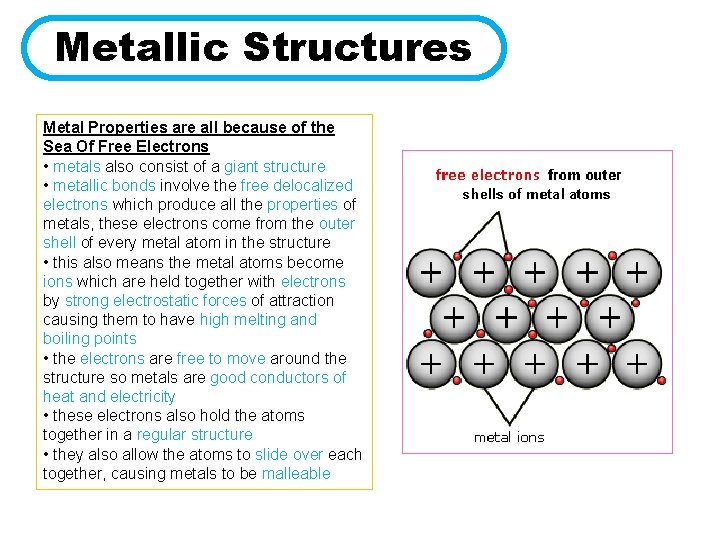
Metallic Structures Metal Properties are all because of the Sea Of Free Electrons • metals also consist of a giant structure • metallic bonds involve the free delocalized electrons which produce all the properties of metals, these electrons come from the outer shell of every metal atom in the structure • this also means the metal atoms become ions which are held together with electrons by strong electrostatic forces of attraction causing them to have high melting and boiling points • the electrons are free to move around the structure so metals are good conductors of heat and electricity • these electrons also hold the atoms together in a regular structure • they also allow the atoms to slide over each together, causing metals to be malleable
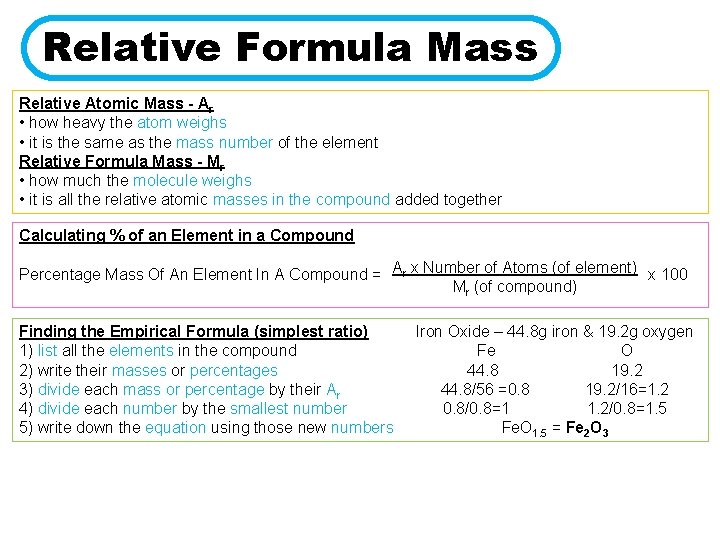
Relative Formula Mass Relative Atomic Mass - Ar • how heavy the atom weighs • it is the same as the mass number of the element Relative Formula Mass - Mr • how much the molecule weighs • it is all the relative atomic masses in the compound added together Calculating % of an Element in a Compound Percentage Mass Of An Element In A Compound = Ar x Number of Atoms (of element) x 100 Mr (of compound) Finding the Empirical Formula (simplest ratio) 1) list all the elements in the compound 2) write their masses or percentages 3) divide each mass or percentage by their Ar 4) divide each number by the smallest number 5) write down the equation using those new numbers Iron Oxide – 44. 8 g iron & 19. 2 g oxygen Fe O 44. 8 19. 2 44. 8/56 =0. 8 19. 2/16=1. 2 0. 8/0. 8=1 1. 2/0. 8=1. 5 Fe. O 1. 5 = Fe 2 O 3
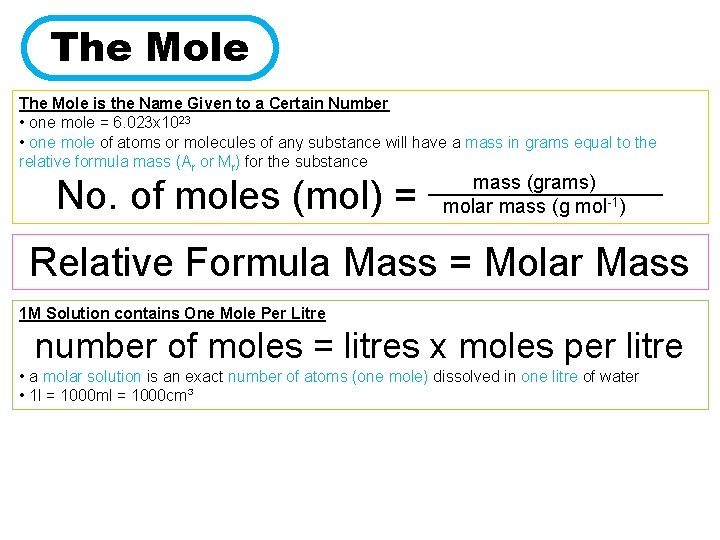
The Mole is the Name Given to a Certain Number • one mole = 6. 023 x 1023 • one mole of atoms or molecules of any substance will have a mass in grams equal to the relative formula mass (Ar or Mr) for the substance No. of moles (mol) = mass (grams) ________ molar mass (g mol-1) Relative Formula Mass = Molar Mass 1 M Solution contains One Mole Per Litre number of moles = litres x moles per litre • a molar solution is an exact number of atoms (one mole) dissolved in one litre of water • 1 l = 1000 ml = 1000 cm³
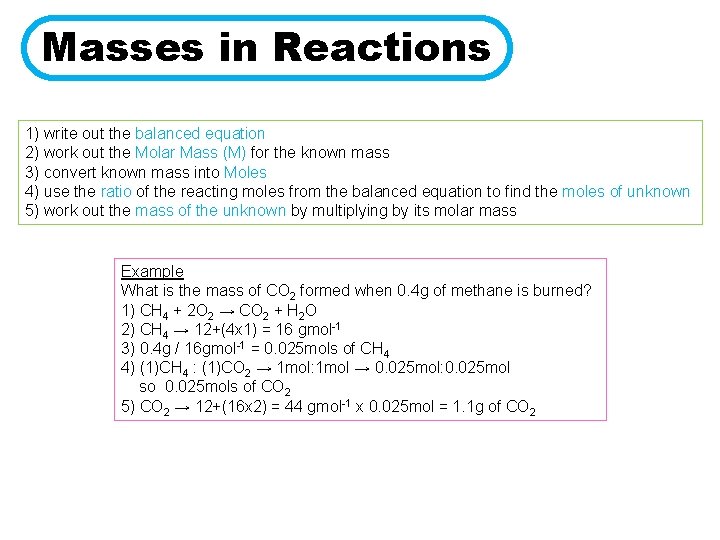
Masses in Reactions 1) write out the balanced equation 2) work out the Molar Mass (M) for the known mass 3) convert known mass into Moles 4) use the ratio of the reacting moles from the balanced equation to find the moles of unknown 5) work out the mass of the unknown by multiplying by its molar mass Example What is the mass of CO 2 formed when 0. 4 g of methane is burned? 1) CH 4 + 2 O 2 → CO 2 + H 2 O 2) CH 4 → 12+(4 x 1) = 16 gmol-1 3) 0. 4 g / 16 gmol-1 = 0. 025 mols of CH 4 4) (1)CH 4 : (1)CO 2 → 1 mol: 1 mol → 0. 025 mol: 0. 025 mol so 0. 025 mols of CO 2 5) CO 2 → 12+(16 x 2) = 44 gmol-1 x 0. 025 mol = 1. 1 g of CO 2

Atom Economy is the Percentage Of Reactant Changed To Useful Products atom economy = total Mr or useful products _________ total Mr of reactants x 100 • reactions with low atom economy use up resources very quickly and make lots of waste material, this makes these reactions unsustainable • low atom economy reaction aren’t usually profitable as raw materials are expensive to buy and waste products can be expensive to remove, dispose of responsibly • the best way to solve this problem is to find a use for the waste products • reaction with the highest atom economy are the ones that only have one product they have an atom economy of 100%
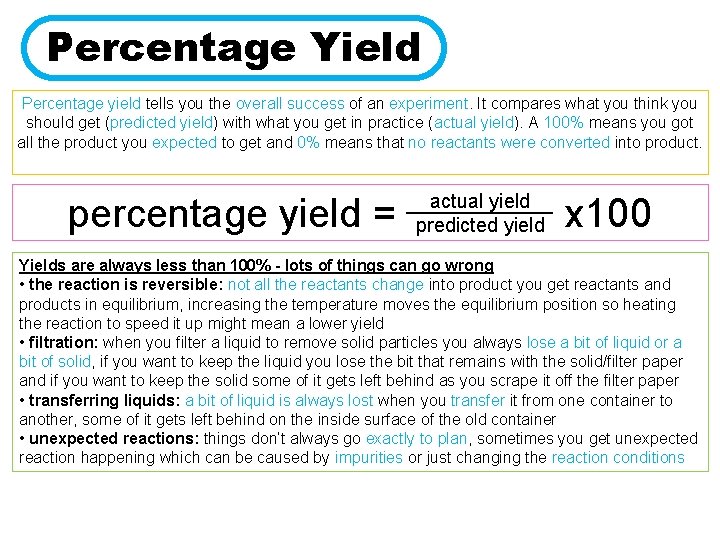
Percentage Yield Percentage yield tells you the overall success of an experiment. It compares what you think you should get (predicted yield) with what you get in practice (actual yield). A 100% means you got all the product you expected to get and 0% means that no reactants were converted into product. percentage yield = actual yield _____ predicted yield x 100 Yields are always less than 100% - lots of things can go wrong • the reaction is reversible: not all the reactants change into product you get reactants and products in equilibrium, increasing the temperature moves the equilibrium position so heating the reaction to speed it up might mean a lower yield • filtration: when you filter a liquid to remove solid particles you always lose a bit of liquid or a bit of solid, if you want to keep the liquid you lose the bit that remains with the solid/filter paper and if you want to keep the solid some of it gets left behind as you scrape it off the filter paper • transferring liquids: a bit of liquid is always lost when you transfer it from one container to another, some of it gets left behind on the inside surface of the old container • unexpected reactions: things don’t always go exactly to plan, sometimes you get unexpected reaction happening which can be caused by impurities or just changing the reaction conditions

RATES OF REACTION
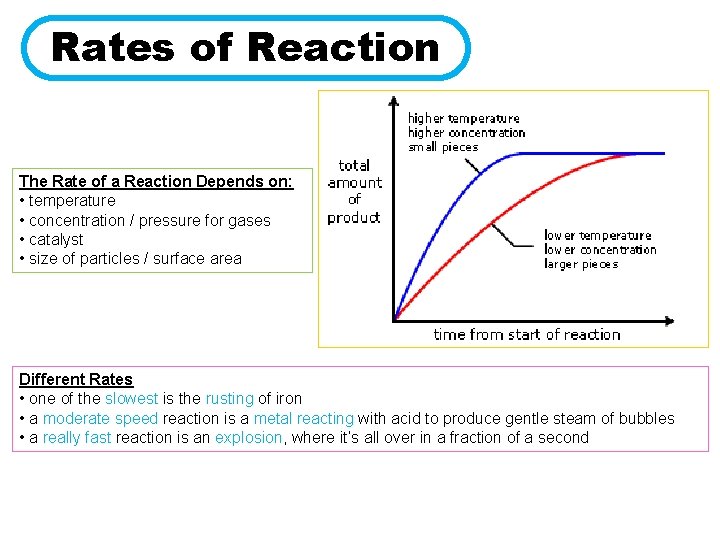
Rates of Reaction The Rate of a Reaction Depends on: • temperature • concentration / pressure for gases • catalyst • size of particles / surface area Different Rates • one of the slowest is the rusting of iron • a moderate speed reaction is a metal reacting with acid to produce gentle steam of bubbles • a really fast reaction is an explosion, where it’s all over in a fraction of a second
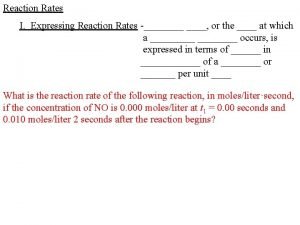
Measuring Rates rate of reaction = amount of reactant used or product formed _______________ time The speed of reaction can be observed either by how quickly the reactants are used up or how quickly the products are formed. It’s usually a lot easier to measure the products forming. Precipitation • when the product of the reaction is a precipitate, a solid is formed which clouds the solution • observe a marker through the solution and measure how long it takes for it to disappear • the quicker the marker disappears, the quicker the reaction • only works for reactions where the initial solution is see through and the result is subjective (based on someone's opinion) Change in Mass (usually gas given off) • measuring the speed of a reaction that produces a gas can be carried out on a mass balance • as the gas is released the mass disappearing is easily measured on the balance • the quicker the reading on the balance drops, the faster the reaction • easy to plot the results and is the most accurate The Volume of Gas Given Off • involves the use of a gas syringe to measure the volume of gas given off • the more gas given off during a given time interval the faster the reaction • a graph of gas volume against time elapsed could be plotted to give a rate of reaction graph • gas syringes usually give volumes accurate to the nearest mililitre, so they’re quite accurate

Collision Theory The rate of reaction depends on how often and how hard the reacting particles collide with each other. Particles have to collide in order to react and they have to collide hard enough with enough energy. Faster Collisions Increase the Rate of Reaction Faster collisions are only caused by increasing the temperature. At a high temperature there will be more particles colliding with enough energy to make the reaction happen. This initial energy is known as the activation energy and it’s needed to break down initial bonds. More Collision Increases the Rate of Reaction Higher Temperature • when the temperature is increased the particles all move quicker, if they’re moving quicker, they’re going to have more collisions Higher Concentration or Pressure • if the solution is more concentrated there are more particles in a certain volume and so more particles mean more collisions • increasing pressure in a gas means the particles are closer together so there are going to be more collisions Larger Surface Area • increasing the surface area means the particles around it in the solution have more area to work and more atoms available for collision, so there’ll be more collisions Catalysts • it works by providing a surface to bring reactants together and weakens the bonds, they increase the number of successful collisions by lowering the activation energy
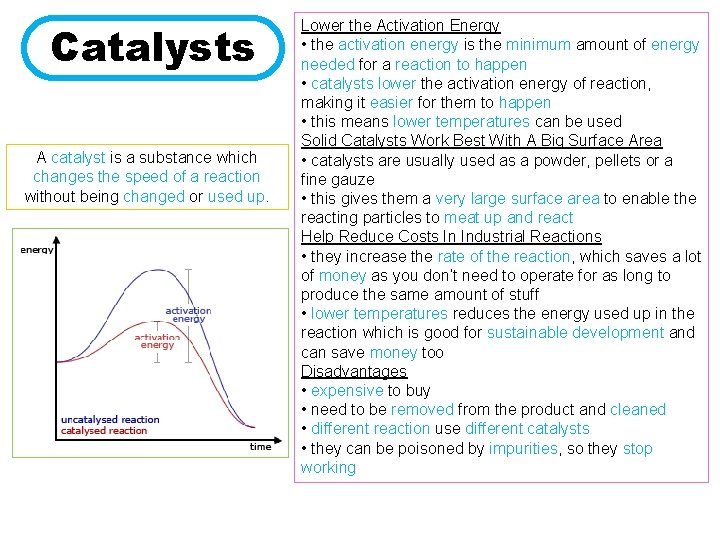
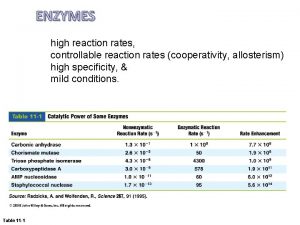
Catalysts A catalyst is a substance which changes the speed of a reaction without being changed or used up. Lower the Activation Energy • the activation energy is the minimum amount of energy needed for a reaction to happen • catalysts lower the activation energy of reaction, making it easier for them to happen • this means lower temperatures can be used Solid Catalysts Work Best With A Big Surface Area • catalysts are usually used as a powder, pellets or a fine gauze • this gives them a very large surface area to enable the reacting particles to meat up and react Help Reduce Costs In Industrial Reactions • they increase the rate of the reaction, which saves a lot of money as you don’t need to operate for as long to produce the same amount of stuff • lower temperatures reduces the energy used up in the reaction which is good for sustainable development and can save money too Disadvantages • expensive to buy • need to be removed from the product and cleaned • different reaction use different catalysts • they can be poisoned by impurities, so they stop working
Energy Transfer In Reactions Whenever chemical reactions happen energy is usually transferred to or from the surroundings. In an Exothermic Reaction Heat is Given Out Exothermic reactions transfer energy to the surroundings, usually in the form of heat and usually shown by a rise in temperature. Energy is released when new bonds are made so it is exothermic. Burning Fuels • the best example of an exothermic reaction is burning fuels which is also called combustion • this gives out a lot of heat Neutralization Reactions • neutralization reaction between acids and alkalis are also exothermic Oxidation Reactions • many oxidation (gaining oxygen) reaction are exothermic In an Endothermic Reaction Heat is Taken In Endothermic reactions take in energy from the surroundings, usually in the form of heat and usually shown by a fall in temperature. Energy must be supplied to break bonds so it is endothermic. Thermal Decomposition • heat must be supplied to make the compound decompose and break down
Reversible Reactions Reversible reactions are where the products of the reaction can react with each other and convert back to the original reactants. Dynamic Equilibrium • if a reversible reaction takes place in a closed system then a state of equilibrium will be reached • equilibrium means that the relative (%) quantities of reactants and products will reach a certain balance and stay there • a closed system means that none of the reactants or products can escape • the reaction are still taking place in both directions, but the overall effect is nil because the forward and reverse reaction cancel each other out • the reactions are taking place at exactly the same rate in both directions Changing Temperature & Pressure To Get More Product In a reversible reaction the position of equilibrium depends very strongly on the temperature and pressure surrounding the reaction. If you deliberately alter the temperature and pressure you can move the position if equilibrium to give more product and less reactants. Temperature • all reactions are exothermic in one direction and endothermic in the other • if you raise the temperature, the endothermic reaction will increase to use up the extra heat • if you reduce the temperature, the exothermic reaction will increase to give out more heat Pressure • if you raise the pressure it will encourage the reaction which produce less volume to reduce the pressure • if you lower the pressure it will encourage the reaction which produces more volume to increase the pressure
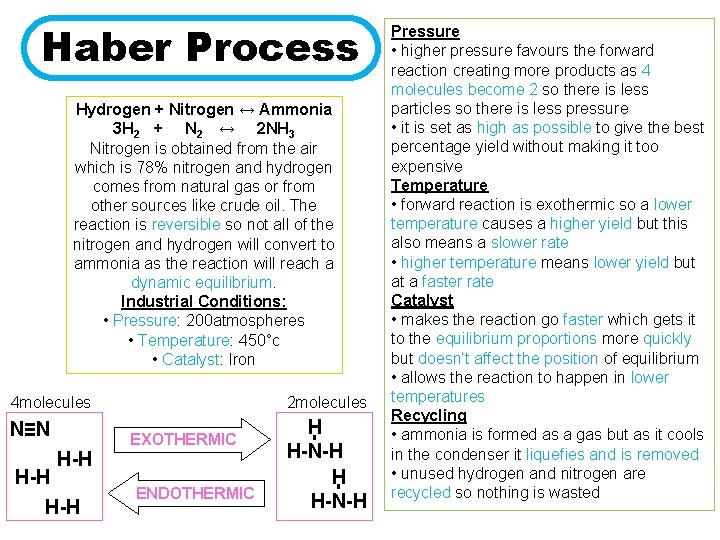
Haber Process Hydrogen + Nitrogen ↔ Ammonia 3 H 2 + N 2 ↔ 2 NH 3 Nitrogen is obtained from the air which is 78% nitrogen and hydrogen comes from natural gas or from other sources like crude oil. The reaction is reversible so not all of the nitrogen and hydrogen will convert to ammonia as the reaction will reach a dynamic equilibrium. Industrial Conditions: • Pressure: 200 atmospheres • Temperature: 450°c • Catalyst: Iron 4 molecules H-H ENDOTHERMIC H H-N-H - H-H EXOTHERMIC - N≡N 2 molecules Pressure • higher pressure favours the forward reaction creating more products as 4 molecules become 2 so there is less particles so there is less pressure • it is set as high as possible to give the best percentage yield without making it too expensive Temperature • forward reaction is exothermic so a lower temperature causes a higher yield but this also means a slower rate • higher temperature means lower yield but at a faster rate Catalyst • makes the reaction go faster which gets it to the equilibrium proportions more quickly but doesn’t affect the position of equilibrium • allows the reaction to happen in lower temperatures Recycling • ammonia is formed as a gas but as it cools in the condenser it liquefies and is removed • unused hydrogen and nitrogen are recycled so nothing is wasted

USING IONS IN SOLUTION
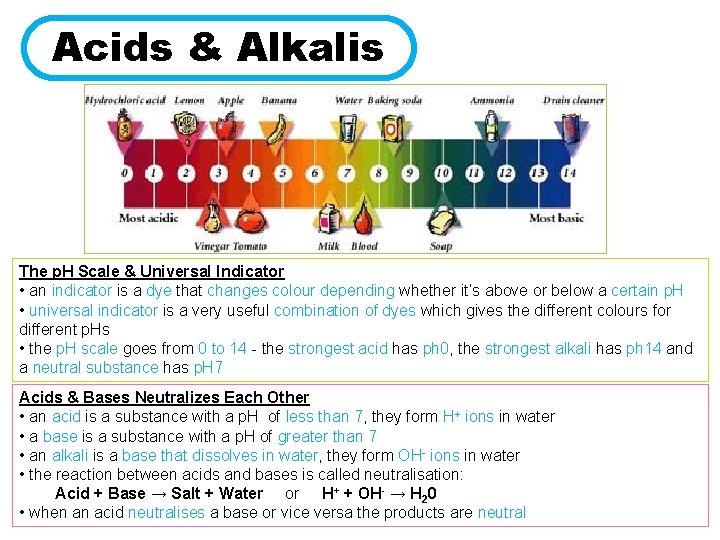
Acids & Alkalis The p. H Scale & Universal Indicator • an indicator is a dye that changes colour depending whether it’s above or below a certain p. H • universal indicator is a very useful combination of dyes which gives the different colours for different p. Hs • the p. H scale goes from 0 to 14 - the strongest acid has ph 0, the strongest alkali has ph 14 and a neutral substance has p. H 7 Acids & Bases Neutralizes Each Other • an acid is a substance with a p. H of less than 7, they form H+ ions in water • a base is a substance with a p. H of greater than 7 • an alkali is a base that dissolves in water, they form OH- ions in water • the reaction between acids and bases is called neutralisation: Acid + Base → Salt + Water or H+ + OH- → H 20 • when an acid neutralises a base or vice versa the products are neutral
Acids & Metals Acid + Metal → Salt + Hydrogen • the more reactive the metal, the faster the reaction will go • copper doesn’t react with dilute acids at all as it’s less reactive than hydrogen • the speed of reaction is indicated by the rate at which the bubbles of hydrogen are given off • the hydrogen is confirmed by the squeaky pop test • the name of the salt produced depends on which metal is used and which acid is used Hydrochloric Acid Always Produces Chloride Salts: • magnesium + hydrochloric acid → magnesium chloride + hydrogen • aluminium + hydrochloric acid → aliminium chloride + hydrogen • zinc + hydrochloric acid → zinc chloride + hydrogen Sulfuric Acid Always Produces Sulfate Salts: • magnesium + sulfuric acid → magnesium sulfate + hydrogen • aluminium + sulfuric acid → aliminium sulfate + hydrogen • zinc + sulfuric acid → zinc sulfate + hydrogen Nitric Acid Produces Nitrate Salts When Neutralised But: • nitric acid reacts with fine alkalis to produce nitrates, but with metals it can produce nitrogen oxide instead

Oxides & Hydroxides Acid + Metal Oxide/Hydroxide→ Salt + Water They are neutralisation reactions: • hydrochloric acid + copper oxide → copper chloride + water • hydrochloric acid + sodium hydroxide → sodium chloride + water • sulfuric acid + zinc oxide → zinc sulfate + water • sulfuric acid + calcium hydroxide → calcium sulfate + water • nitric acid + magnesium oxide → magnesium nitrate + water • nitric acid + potassium hydroxide → potassium nitrate + water • ammonia can be neutralised with nitric acid to make ferlisers • it dissolves in water to make an alkaline solution • when it reacts with nitric acid, you get a neutral salt • ammonia + nitric acid → ammonium nitrate • this is different from most neuralisation reactions because no water is produced • ammonium nitrates is an especially good fertiliser because it has nitrogen from two sources, plants need this to make proteins

Making Salts Most chlorides, sulfates and nitrates are soluble in water. Most oxides , hydroxides and carbonates are insoluble in water. If both the base and salt are soluble, you have to add exactly the right amount of base to neutralise the acid, by using an indicator. Making Soluble Salts from Insoluble Bases • pick the right acid and a metal carbonate or metal hydroxide that is insoluble • add the carbonate or hydroxide to the acid until all the acid is neutralised • filter the excess carbonate or hydroxide and evaporate the water • you are left with a pure dry salt • copper carbonate + nitric acid → copper nitrate + carbon dioxide + water Making Insoluble Salts – Precipitation Reaction • if the salt you want to make it insoluble you can use a precipitation reaction • mix the right acid and nitrate – lead chloride → hydrochloric acid + lead nitrate • once the salt has precipitates out, filter it from the solution, wash it and dry it on filter paper • precipitation reactions can be used to remove poisonous ions from drinking water
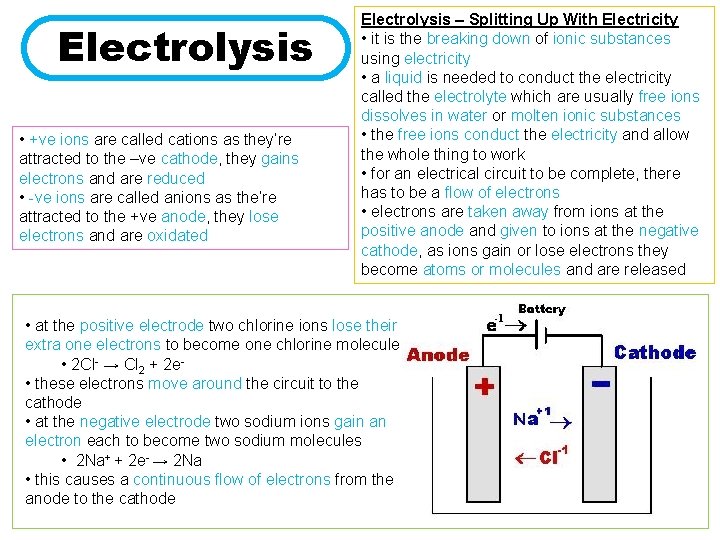
Electrolysis • +ve ions are called cations as they’re attracted to the –ve cathode, they gains electrons and are reduced • -ve ions are called anions as the’re attracted to the +ve anode, they lose electrons and are oxidated Electrolysis – Splitting Up With Electricity • it is the breaking down of ionic substances using electricity • a liquid is needed to conduct the electricity called the electrolyte which are usually free ions dissolves in water or molten ionic substances • the free ions conduct the electricity and allow the whole thing to work • for an electrical circuit to be complete, there has to be a flow of electrons • electrons are taken away from ions at the positive anode and given to ions at the negative cathode, as ions gain or lose electrons they become atoms or molecules and are released • at the positive electrode two chlorine ions lose their extra one electrons to become one chlorine molecule • 2 Cl- → Cl 2 + 2 e • these electrons move around the circuit to the cathode • at the negative electrode two sodium ions gain an electron each to become two sodium molecules • 2 Na+ + 2 e- → 2 Na • this causes a continuous flow of electrons from the anode to the cathode
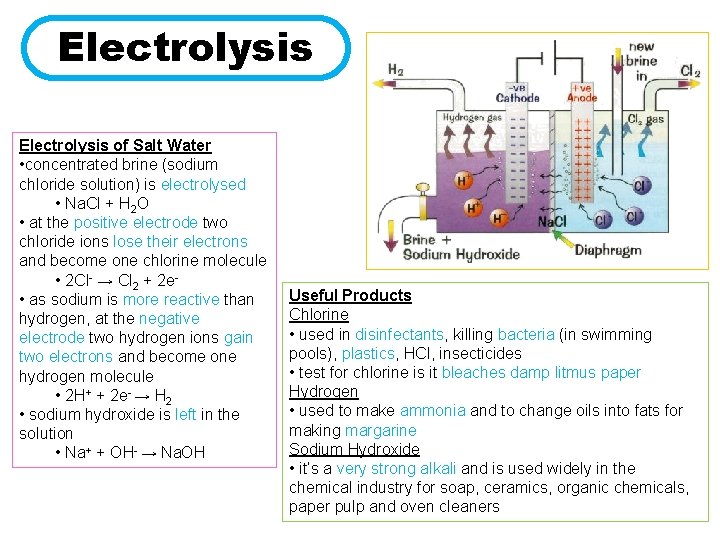
Electrolysis of Salt Water • concentrated brine (sodium chloride solution) is electrolysed • Na. Cl + H 2 O • at the positive electrode two chloride ions lose their electrons and become one chlorine molecule • 2 Cl- → Cl 2 + 2 e • as sodium is more reactive than hydrogen, at the negative electrode two hydrogen ions gain two electrons and become one hydrogen molecule • 2 H+ + 2 e- → H 2 • sodium hydroxide is left in the solution • Na+ + OH- → Na. OH Useful Products Chlorine • used in disinfectants, killing bacteria (in swimming pools), plastics, HCl, insecticides • test for chlorine is it bleaches damp litmus paper Hydrogen • used to make ammonia and to change oils into fats for making margarine Sodium Hydroxide • it’s a very strong alkali and is used widely in the chemical industry for soap, ceramics, organic chemicals, paper pulp and oven cleaners
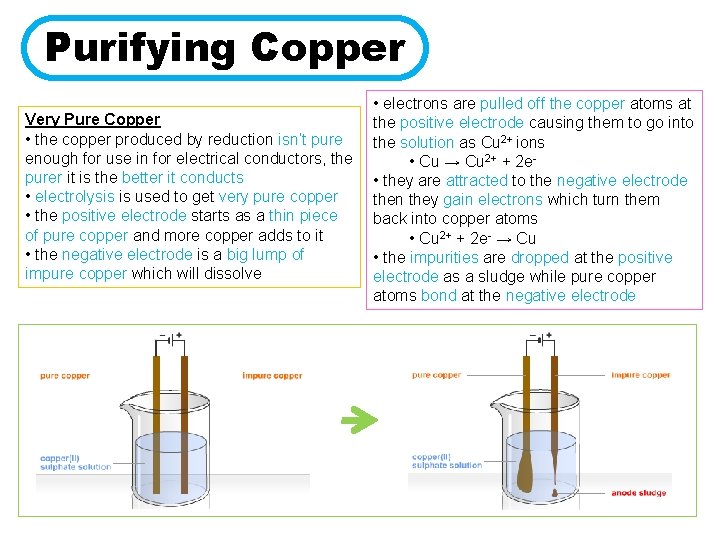
Purifying Copper Very Pure Copper • the copper produced by reduction isn’t pure enough for use in for electrical conductors, the purer it is the better it conducts • electrolysis is used to get very pure copper • the positive electrode starts as a thin piece of pure copper and more copper adds to it • the negative electrode is a big lump of impure copper which will dissolve • electrons are pulled off the copper atoms at the positive electrode causing them to go into the solution as Cu 2+ ions • Cu → Cu 2+ + 2 e • they are attracted to the negative electrode then they gain electrons which turn them back into copper atoms • Cu 2+ + 2 e- → Cu • the impurities are dropped at the positive electrode as a sludge while pure copper atoms bond at the negative electrode
- Slides: 32