The Ideal Gas Law PV n RT Adds











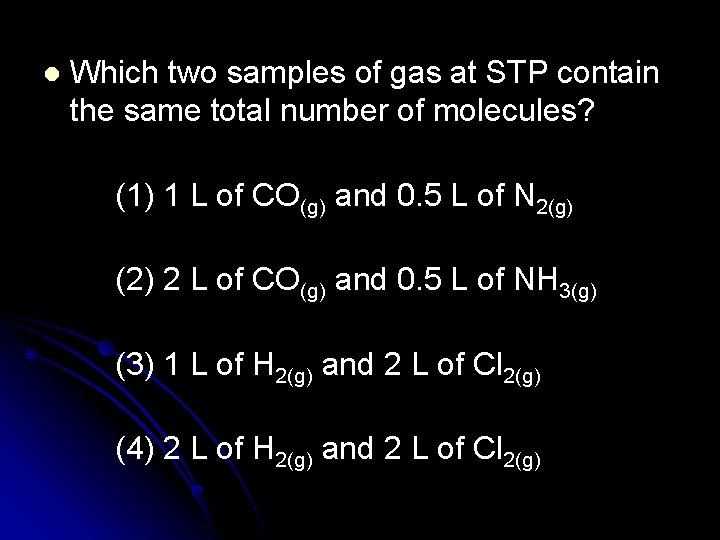

- Slides: 37

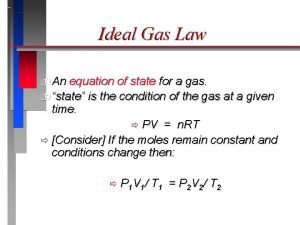
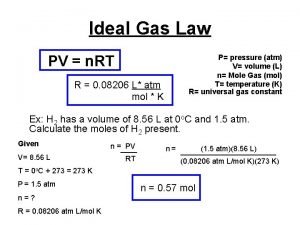
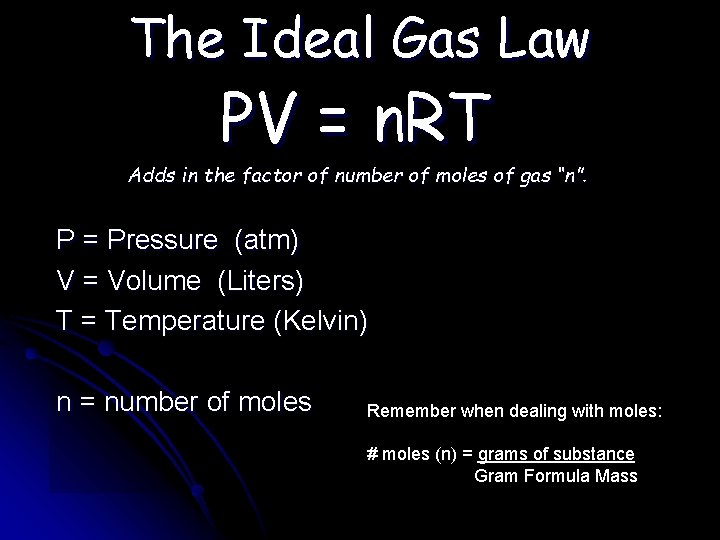
The Ideal Gas Law PV = n. RT Adds in the factor of number of moles of gas “n”. P = Pressure (atm) V = Volume (Liters) T = Temperature (Kelvin) n = number of moles Remember when dealing with moles: # moles (n) = grams of substance Gram Formula Mass

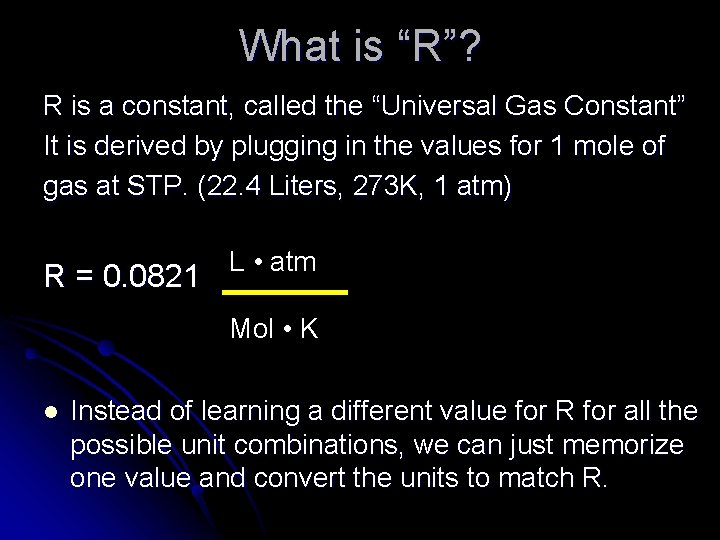
What is “R”? R is a constant, called the “Universal Gas Constant” It is derived by plugging in the values for 1 mole of gas at STP. (22. 4 Liters, 273 K, 1 atm) L • atm R = 0. 0821 Mol • K l Instead of learning a different value for R for all the possible unit combinations, we can just memorize one value and convert the units to match R.

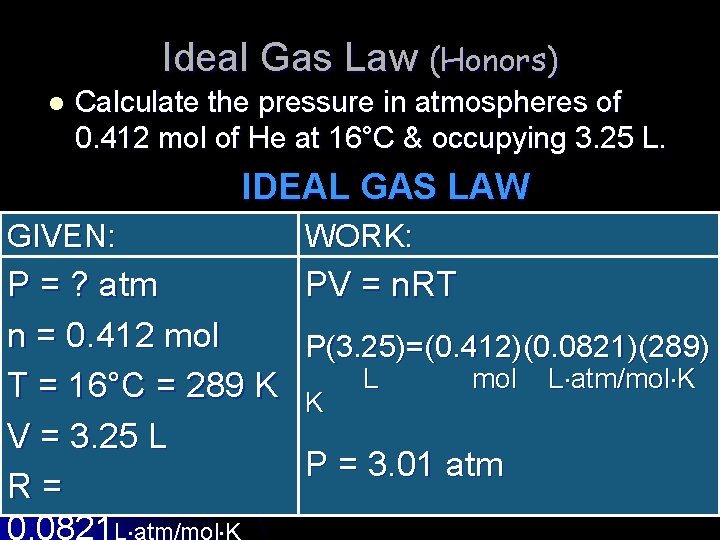
Ideal Gas Law (Honors) l Calculate the pressure in atmospheres of 0. 412 mol of He at 16°C & occupying 3. 25 L. IDEAL GAS LAW GIVEN: WORK: P = ? atm n = 0. 412 mol T = 16°C = 289 K V = 3. 25 L R = 0. 0821 L atm/mol K PV = n. RT P(3. 25)=(0. 412)(0. 0821)(289) L mol L atm/mol K K K P = 3. 01 atm

Example l # 1 Honors Packet l A 9. 81 L cylinder contains 23. 5 moles of nitrogen at 23° C. What pressure is exerted by the gas?

Example l # 2 Honors Packet l A pressure of 850 mm. Hg is exerted by 28. 6 grams of sulfur dioxide at a temp of 40 °C. Calculate the volume of the vessel holding the gas.

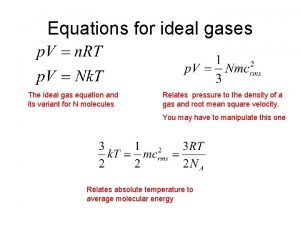
Density is Hidden in this Formula l Density = Mass (g) Volume # moles = Mass (g) Gram formula mass Can you rework PV = n. RT to solve for Density?

Example l # 7 in Honors Packet l What is the density of neon at 40 °C and 1. 23 atmospheres?

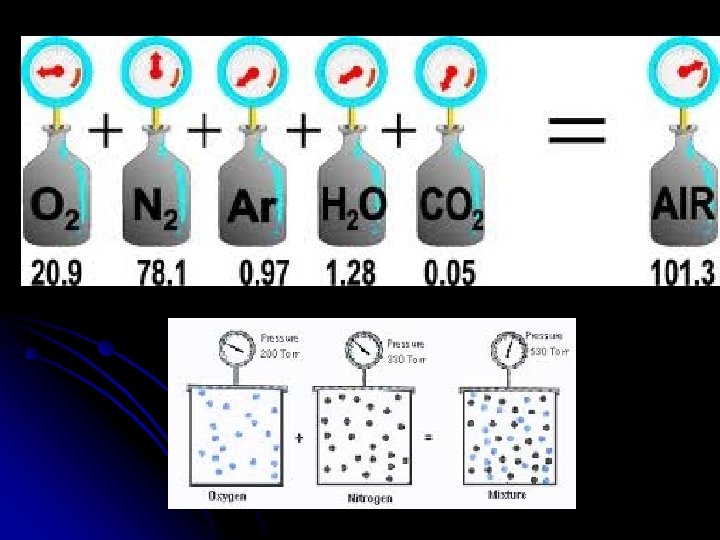
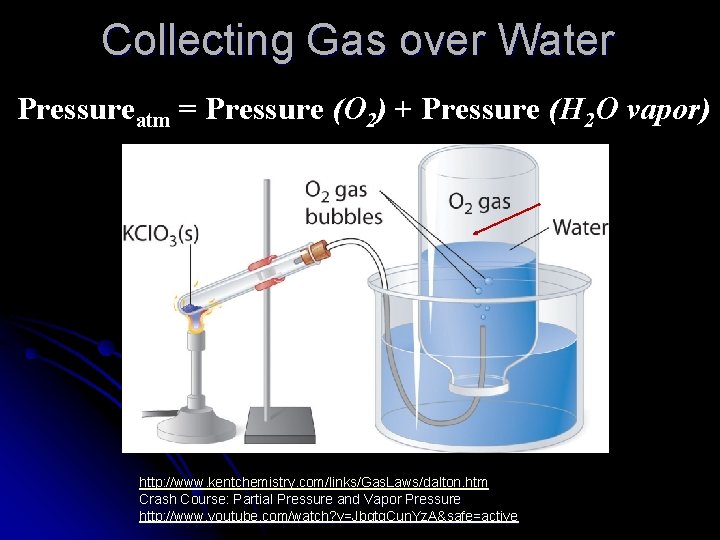
Dalton’s Law of Partial Pressures Ptotal = P 1+P 2+…. l Total pressure of a mixture of gases in a container is the sum of the individual pressures (partial pressures) of each gas, as if each took up the total space alone. l This is often useful when gases are collected “over water”


Collecting Gas over Water Pressureatm = Pressure (O 2) + Pressure (H 2 O vapor) http: //www. kentchemistry. com/links/Gas. Laws/dalton. htm Crash Course: Partial Pressure and Vapor Pressure http: //www. youtube. com/watch? v=Jbqtq. Cun. Yz. A&safe=active

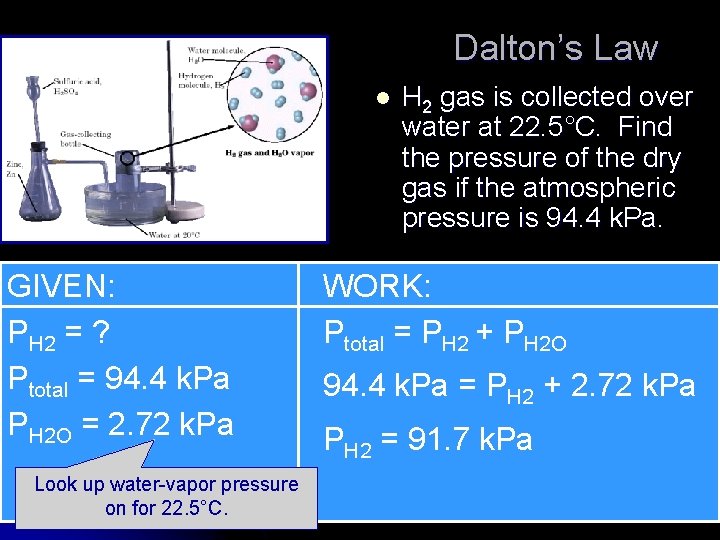
Dalton’s Law l GIVEN: PH 2 = ? Ptotal = 94. 4 k. Pa PH 2 O = 2. 72 k. Pa Look up water-vapor pressure on for 22. 5°C. H 2 gas is collected over water at 22. 5°C. Find the pressure of the dry gas if the atmospheric pressure is 94. 4 k. Pa. WORK: Ptotal = PH 2 + PH 2 O 94. 4 k. Pa = PH 2 + 2. 72 k. Pa PH 2 = 91. 7 k. Pa

Dalton’s Law l A gas is collected over water at a temp of 35. 0°C when the barometric pressure is 742. 0 torr. What is the partial pressure of the dry gas? DALTON’S LAW GIVEN: Pgas = ? Ptotal = 742. 0 torr PH 2 O = 42. 2 torr Look up water-vapor pressure for 35. 0°C. WORK: Ptotal = Pgas + PH 2 O 742. 0 torr = PH 2 + 42. 2 torr Pgas = 699. 8 torr

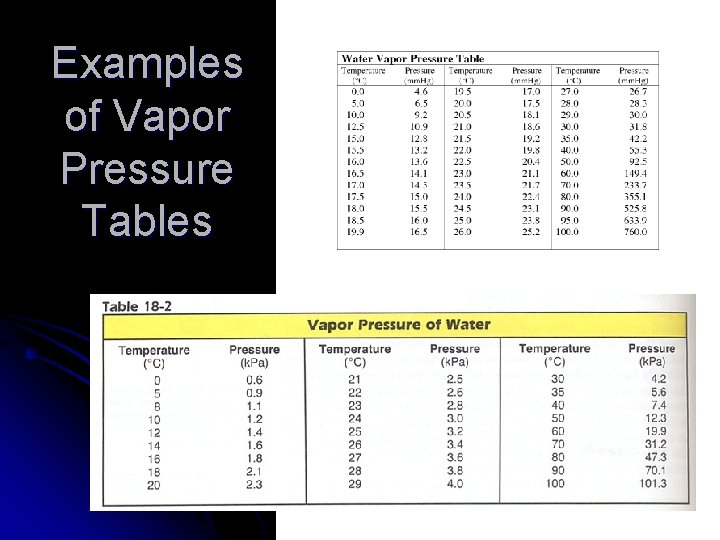
Examples of Vapor Pressure Tables

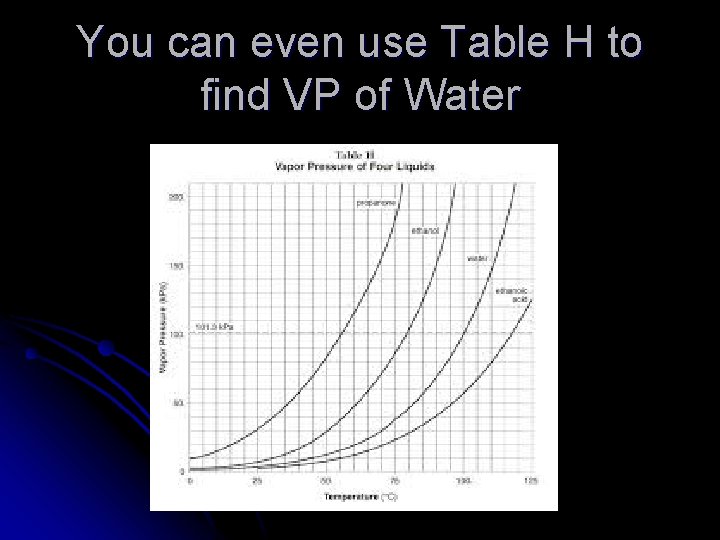
You can even use Table H to find VP of Water

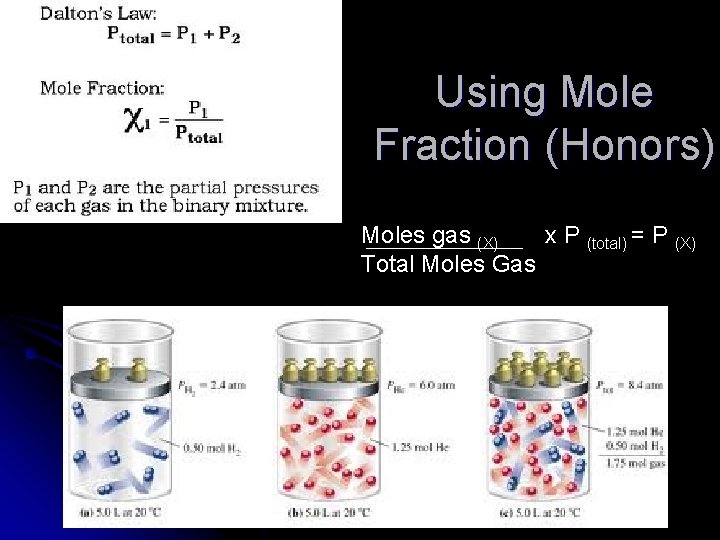
Using Mole Fraction (Honors) Moles gas (X) x P (total) = P (X) Total Moles Gas

Example l # 4 Honors Packet l A mixture of 2. 00 moles H 2, 3. 00 moles NH 3, 4. 00 moles CO 2, and 5. 00 moles N 2 exert a total pressure of 800 mm. Hg. What is the partial pressure of each gas?

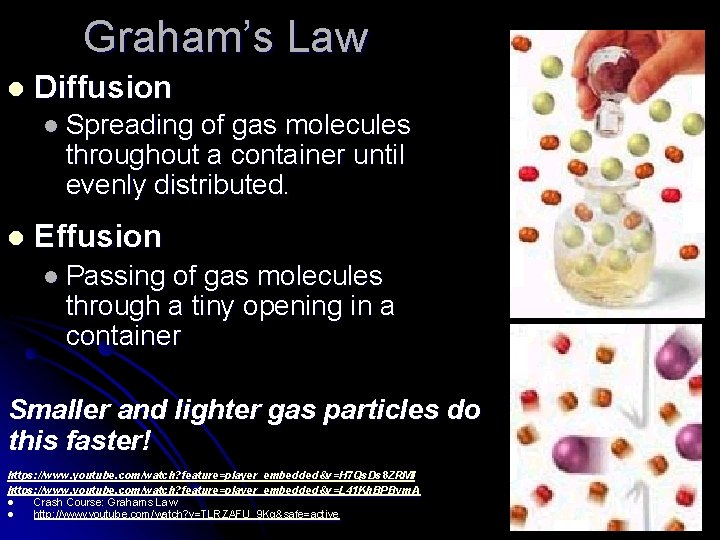
Graham’s Law l Diffusion l Spreading of gas molecules throughout a container until evenly distributed. l Effusion l Passing of gas molecules through a tiny opening in a container Smaller and lighter gas particles do this faster! https: //www. youtube. com/watch? feature=player_embedded&v=H 7 Qs. Ds 8 ZRMI https: //www. youtube. com/watch? feature=player_embedded&v=L 41 Kh. BPBym. A l Crash Course: Grahams Law l http: //www. youtube. com/watch? v=TLRZAFU_9 Kg&safe=active

Graham’s Law l Speed of diffusion/effusion l At the same temp & KE, heavier molecules move more slowly. l Ex: Which of the following gases will diffuse most rapidly? A. ) N 2 B. ) CO 2 C. ) CH 4

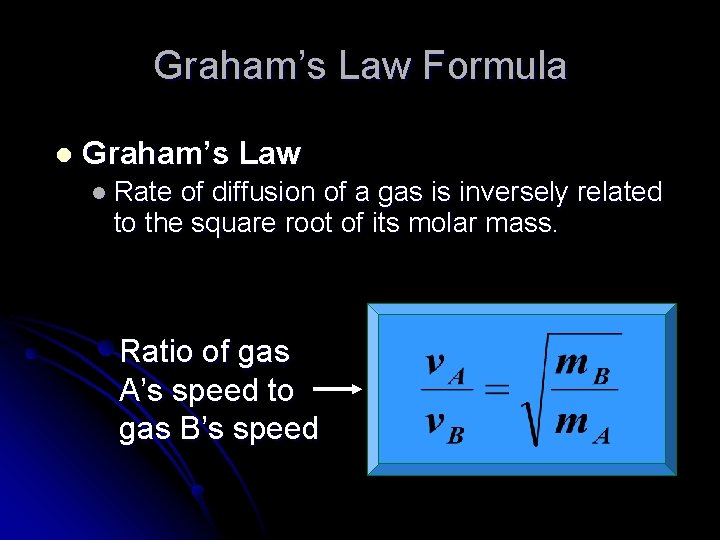
Graham’s Law Formula l Graham’s Law l Rate of diffusion of a gas is inversely related to the square root of its molar mass. Ratio of gas A’s speed to gas B’s speed

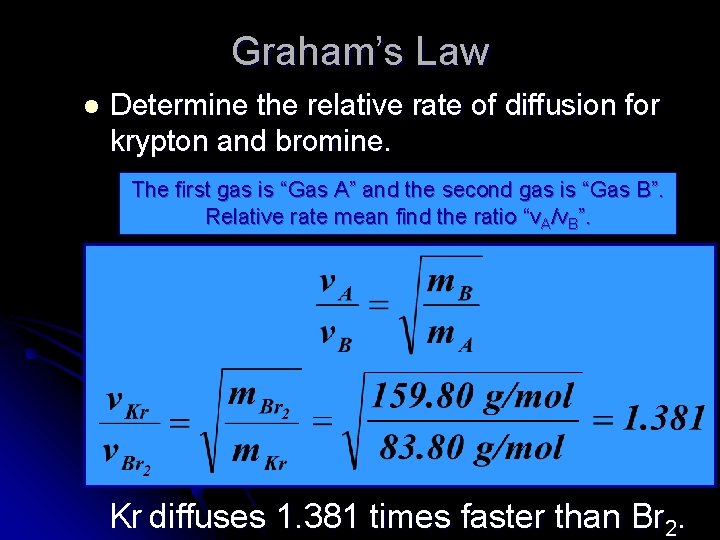
Graham’s Law l Determine the relative rate of diffusion for krypton and bromine. The first gas is “Gas A” and the second gas is “Gas B”. Relative rate mean find the ratio “v. A/v. B”. Kr diffuses 1. 381 times faster than Br 2.

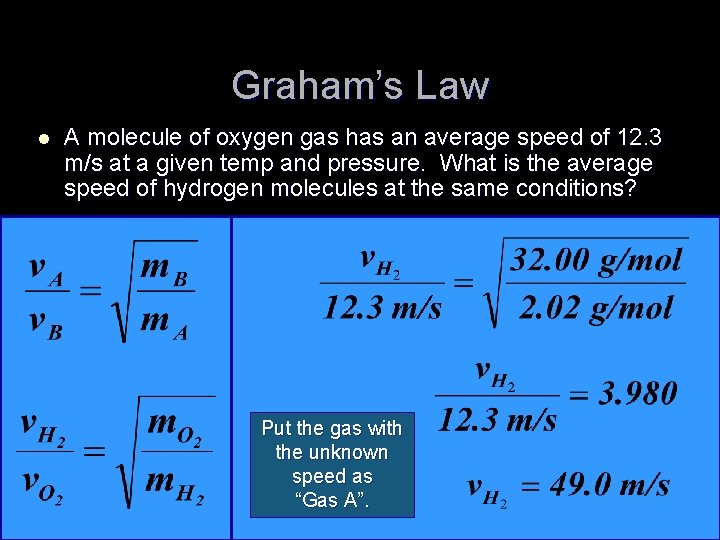
Graham’s Law l A molecule of oxygen gas has an average speed of 12. 3 m/s at a given temp and pressure. What is the average speed of hydrogen molecules at the same conditions? Put the gas with the unknown speed as “Gas A”.

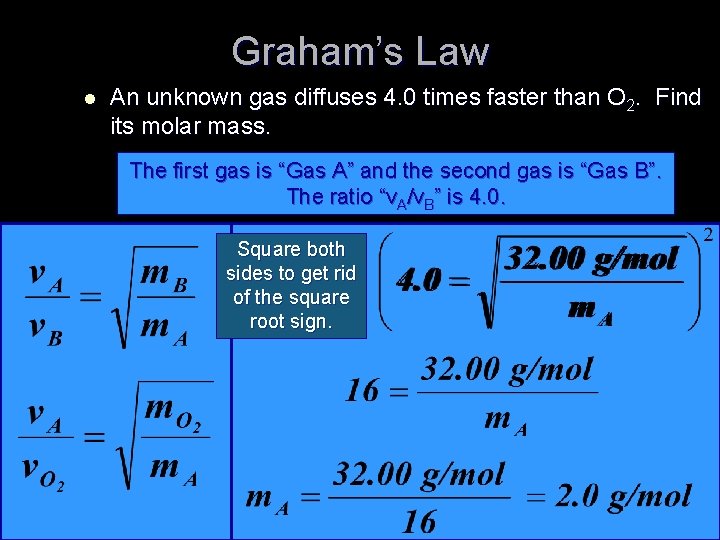
Graham’s Law l An unknown gas diffuses 4. 0 times faster than O 2. Find its molar mass. The first gas is “Gas A” and the second gas is “Gas B”. The ratio “v. A/v. B” is 4. 0. Square both sides to get rid of the square root sign.

Example l # 1 Honors Packet l Under the same conditions of temp and pressure, how many times faster will hydrogen effuse compared to carbon dioxide?

Example l #7 Honors Packet l If CO 2 diffuses from a flask in 30 seconds and an unknown gas diffuses from the same flask in 5 minutes, calculate the molecular weight of this unknown gas.

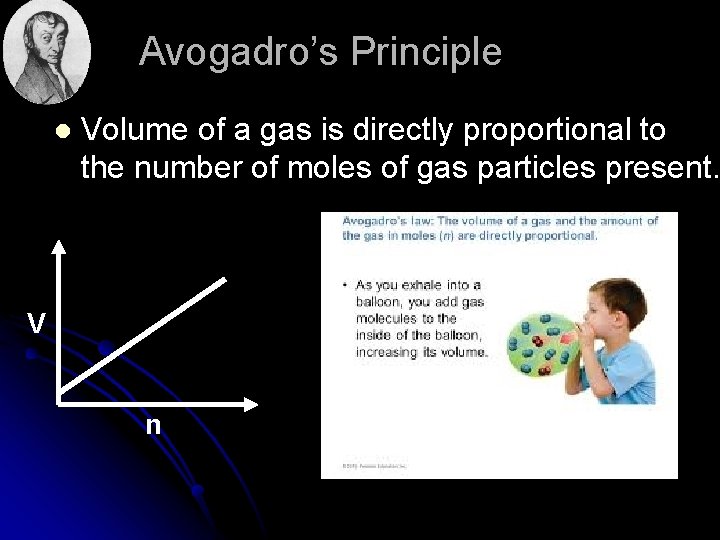
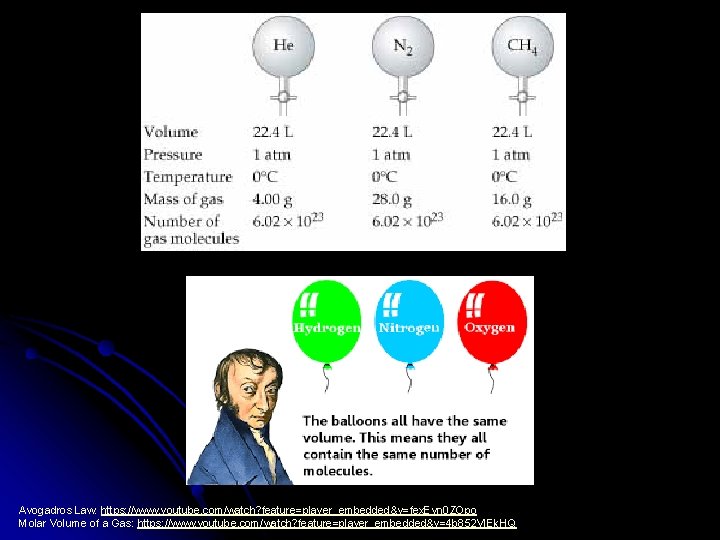
Avogadro’s Principle l Volume of a gas is directly proportional to the number of moles of gas particles present. V n

l Equal volumes of gases contain equal numbers of moles of particles at constant temp & pressure l true for any gas l

Avogadros Law: https: //www. youtube. com/watch? feature=player_embedded&v=fex. Evn 0 ZOpo Molar Volume of a Gas: https: //www. youtube. com/watch? feature=player_embedded&v=4 b 852 VIEk. HQ

Practice Questions l A. At the same temperature and pressure, 1. 0 liter of CO(g) and 1. 0 liter of CO 2(g) have equal masses and the same number of molecules B. different masses and a different number of molecules C. equal volumes and the same number of molecules D. different volumes and a different number of molecules

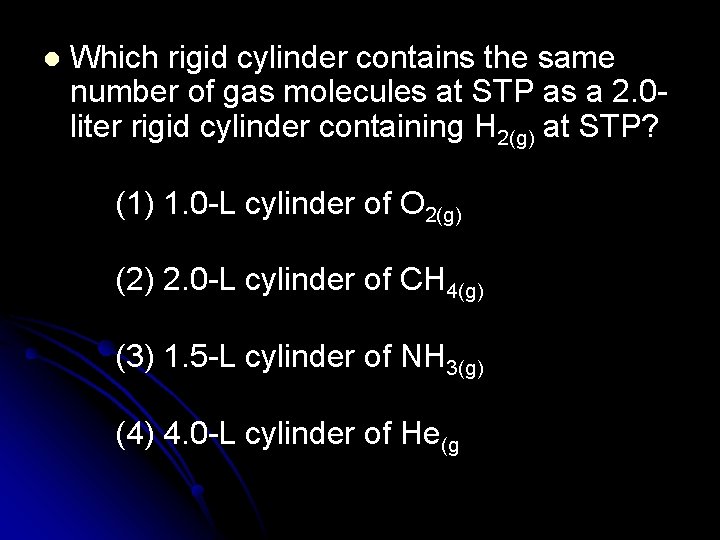
l Which rigid cylinder contains the same number of gas molecules at STP as a 2. 0 liter rigid cylinder containing H 2(g) at STP? (1) 1. 0 -L cylinder of O 2(g) (2) 2. 0 -L cylinder of CH 4(g) (3) 1. 5 -L cylinder of NH 3(g) (4) 4. 0 -L cylinder of He(g
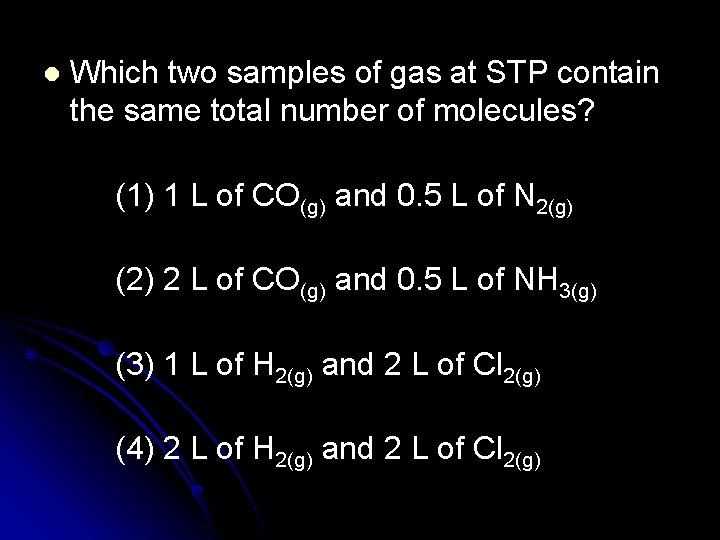
l Which two samples of gas at STP contain the same total number of molecules? (1) 1 L of CO(g) and 0. 5 L of N 2(g) (2) 2 L of CO(g) and 0. 5 L of NH 3(g) (3) 1 L of H 2(g) and 2 L of Cl 2(g) (4) 2 L of H 2(g) and 2 L of Cl 2(g)

Gas Stoichiometry l Moles Liters of a Gas STP - use 22. 4 L/mol l Non-STP - use ideal gas law l l Non-STP Problems l Given liters of gas? l l start with ideal gas law Looking for liters of gas? l start with stoichiometry conv.

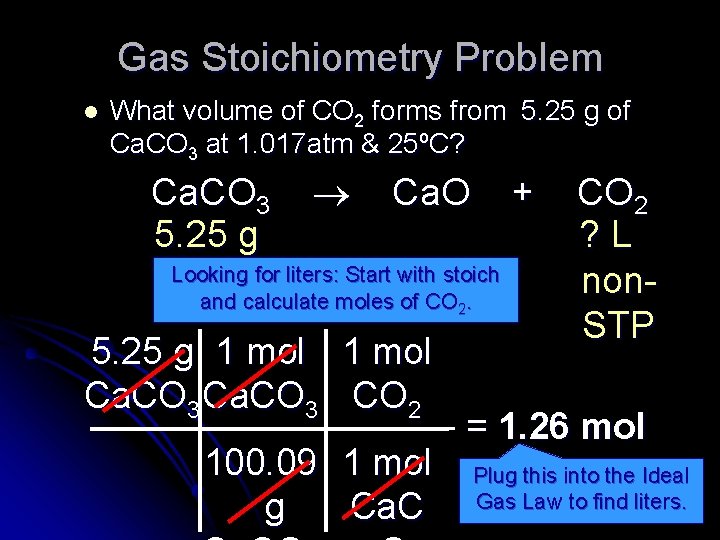
Gas Stoichiometry Problem l What volume of CO 2 forms from 5. 25 g of Ca. CO 3 at 1. 017 atm & 25ºC? Ca. CO 3 Ca. O + CO 2 5. 25 g ? L Looking for liters: Start with stoich nonand calculate moles of CO. STP 5. 25 g 1 mol Ca. CO 3 CO 2 = 1. 26 mol 100. 09 1 mol Plug this into the Ideal CO 2 g Ca. C Gas Law to find liters. 2

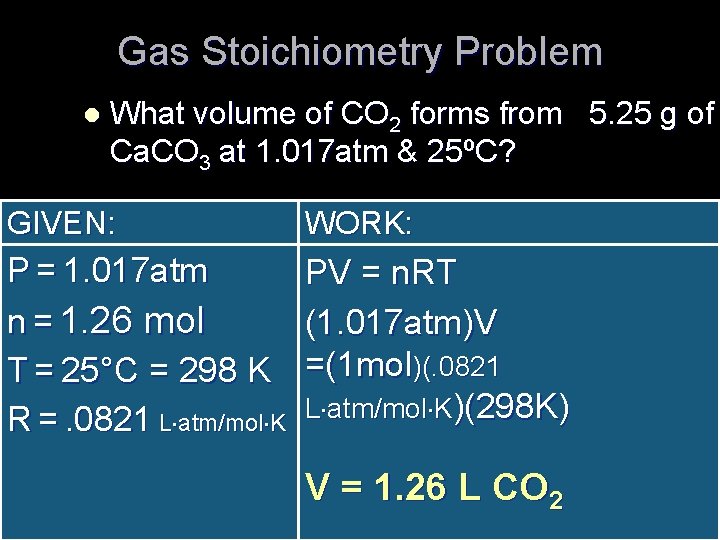
Gas Stoichiometry Problem l What volume of CO 2 forms from 5. 25 g of Ca. CO 3 at 1. 017 atm & 25ºC? GIVEN: WORK: P = 1. 017 atm n = 1. 26 mol T = 25°C = 298 K R =. 0821 L atm/mol K PV = n. RT (1. 017 atm)V =(1 mol)(. 0821 L atm/mol K)(298 K) V = 1. 26 L CO 2

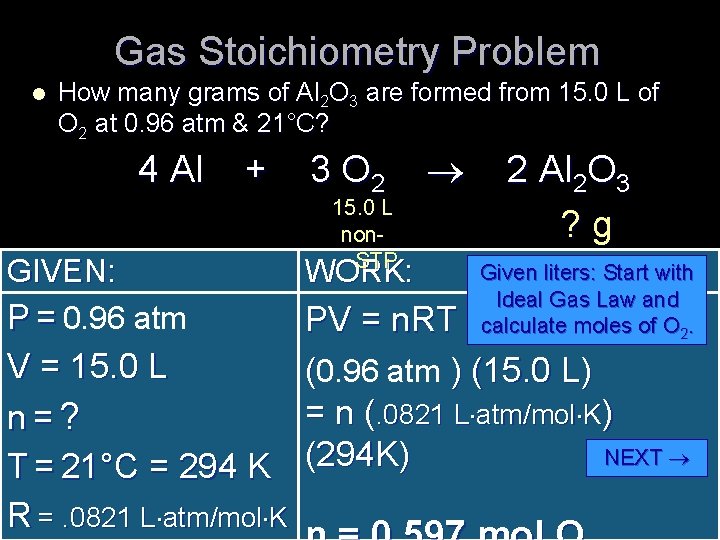
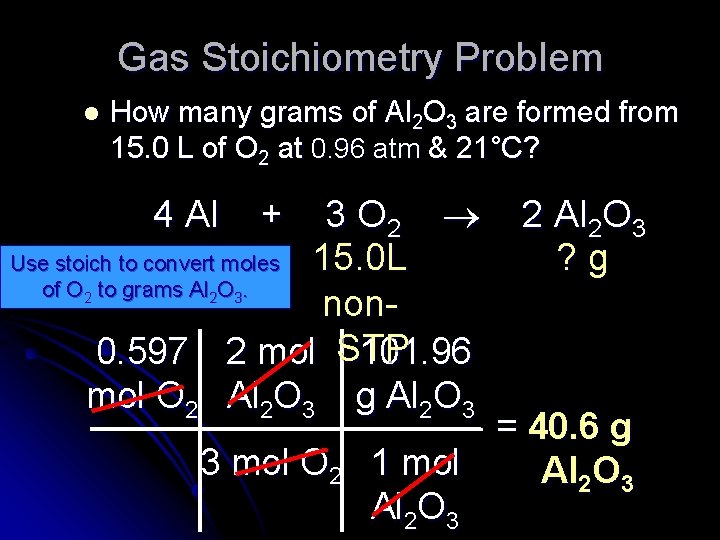
Gas Stoichiometry Problem l How many grams of Al 2 O 3 are formed from 15. 0 L of O 2 at 0. 96 atm & 21°C? 4 Al + 3 O 2 2 Al 2 O 3 15. 0 L ? g non. GIVEN: P = 0. 96 atm STP WORK: Given liters: Start with Ideal Gas Law and calculate moles of O 2. PV = n. RT V = 15. 0 L (0. 96 atm ) (15. 0 L) = n (. 0821 L atm/mol K) n = ? NEXT T = 21°C = 294 K (294 K) R =. 0821 L atm/mol K

Gas Stoichiometry Problem l How many grams of Al 2 O 3 are formed from 15. 0 L of O 2 at 0. 96 atm & 21°C? 4 Al + 3 O 2 2 Al 2 O 3 ? g Use stoich to convert moles 15. 0 L of O to grams Al O. non 0. 597 2 mol STP 101. 96 mol O 2 Al 2 O 3 g Al 2 O 3 = 40. 6 g 3 mol O 2 1 mol Al 2 O 3 2 2 3

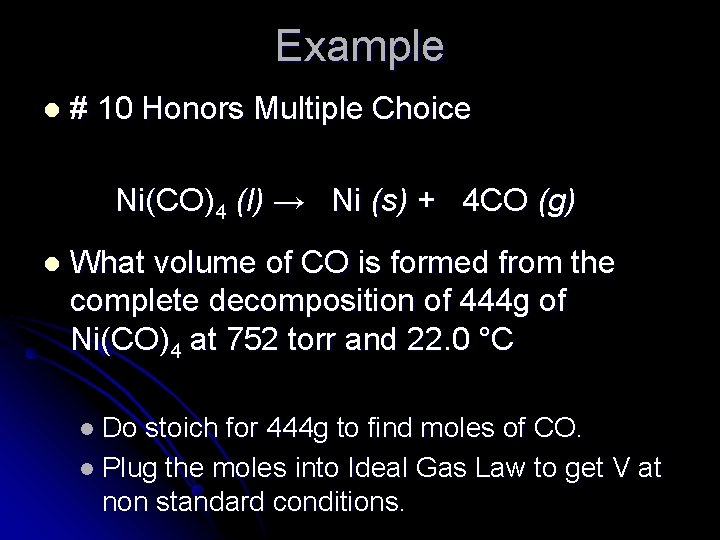
Example l # 10 Honors Multiple Choice Ni(CO)4 (l) → Ni (s) + 4 CO (g) l What volume of CO is formed from the complete decomposition of 444 g of Ni(CO)4 at 752 torr and 22. 0 °C l Do stoich for 444 g to find moles of CO. l Plug the moles into Ideal Gas Law to get V at non standard conditions.

Some Cool Videos l l l Crash Course: Ideal Gas Laws http: //www. youtube. com/watch? v=Bx. US 1 K 7 xu 30&safe=active Crash Course: Ideal Gas Law Problems http: //www. youtube. com/watch? v=8 SRAk. XMu 3 d 0 Crash Course: Real Gases http: //www. youtube. com/watch? v=GIPrs. Wu. Sk. Qc&safe=active
 Ideal gas vs perfect gas
Ideal gas vs perfect gas Imaginary gas
Imaginary gas Gas law
Gas law Sutherland's law
Sutherland's law Difference between ideal gas and real gas
Difference between ideal gas and real gas Unit of pressure
Unit of pressure Gas laws calculations
Gas laws calculations Ideal gas law examples
Ideal gas law examples Which equation agrees with the ideal gas law?
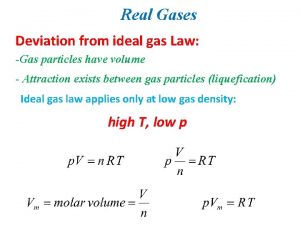
Which equation agrees with the ideal gas law? Deviation from ideal gas
Deviation from ideal gas This is for
This is for Deviations from the ideal gas law
Deviations from the ideal gas law Ideal gas graph
Ideal gas graph Density in ideal gas law
Density in ideal gas law State ideal gas law
State ideal gas law R constant chemistry
R constant chemistry Density equation ideal gas
Density equation ideal gas Ideal gas law formula
Ideal gas law formula How to find density in ideal gas law
How to find density in ideal gas law Ideal gas law to find density
Ideal gas law to find density Entropy of ideal gas
Entropy of ideal gas Pv nrt
Pv nrt Ideal gas law equation example
Ideal gas law equation example Empirical derivation of gas equation
Empirical derivation of gas equation Characteristics of ideal gas
Characteristics of ideal gas Kinetic molecular theory
Kinetic molecular theory Gas variable relationships
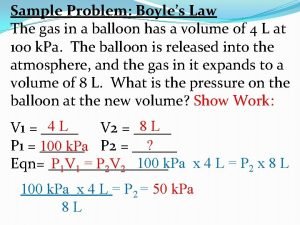
Gas variable relationships Sample problem boyle's law
Sample problem boyle's law If i place 3 moles of n2 and 4 moles of o2 in a 35l
If i place 3 moles of n2 and 4 moles of o2 in a 35l Ideal gas law
Ideal gas law Ideal gas law omni
Ideal gas law omni Mlk poem generator
Mlk poem generator Helium gas constant
Helium gas constant Pv=nrt units
Pv=nrt units Ideal.gas law
Ideal.gas law Boyle's law formula example
Boyle's law formula example First law of thermodynamics for ideal gas
First law of thermodynamics for ideal gas Newton's first law and second law and third law
Newton's first law and second law and third law