Gases Gas Laws Boyles Law The pressure and
Gases Gas Laws
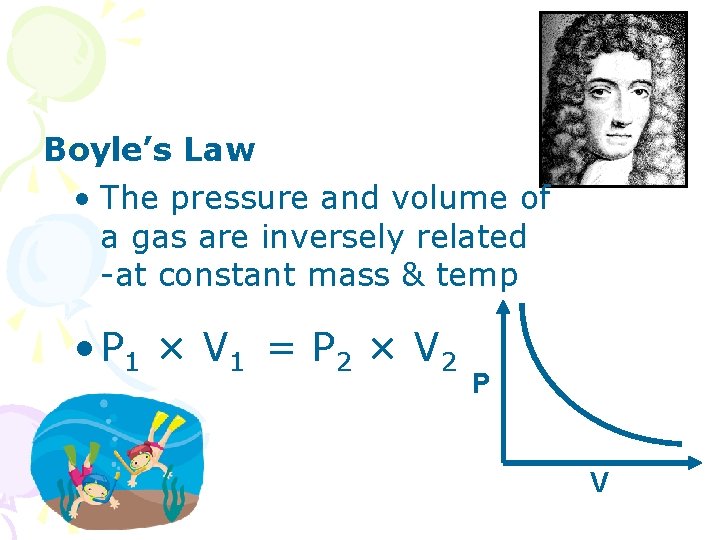
Boyle’s Law • The pressure and volume of a gas are inversely related -at constant mass & temp • P 1 × V 1 = P 2 × V 2 P V
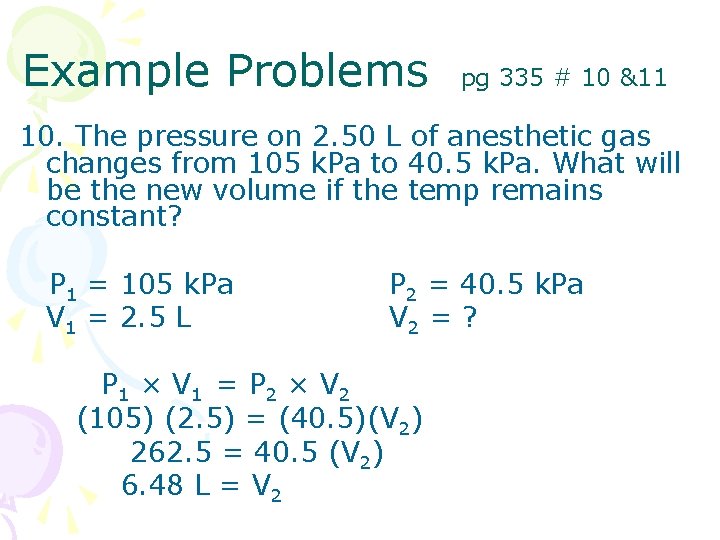
Example Problems pg 335 # 10 &11 10. The pressure on 2. 50 L of anesthetic gas changes from 105 k. Pa to 40. 5 k. Pa. What will be the new volume if the temp remains constant? P 1 = 105 k. Pa V 1 = 2. 5 L P 2 = 40. 5 k. Pa V 2 = ? P 1 × V 1 = P 2 × V 2 (105) (2. 5) = (40. 5)(V 2) 262. 5 = 40. 5 (V 2) 6. 48 L = V 2
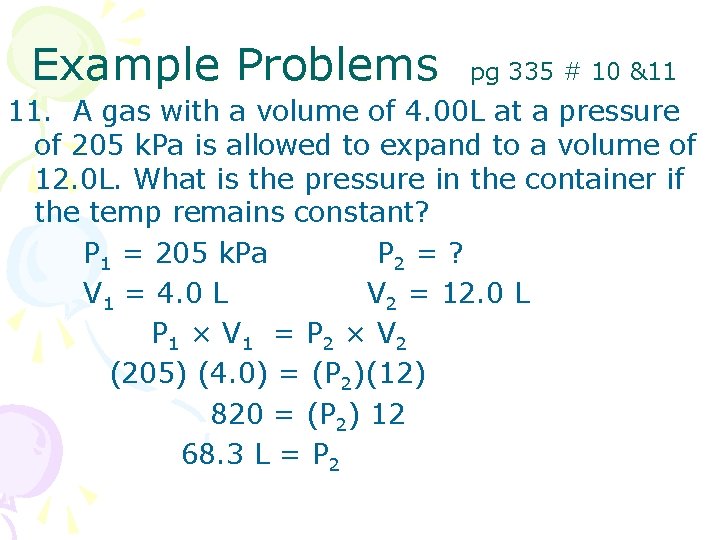
Example Problems pg 335 # 10 &11 11. A gas with a volume of 4. 00 L at a pressure of 205 k. Pa is allowed to expand to a volume of 12. 0 L. What is the pressure in the container if the temp remains constant? P 1 = 205 k. Pa P 2 = ? V 1 = 4. 0 L V 2 = 12. 0 L P 1 × V 1 = P 2 × V 2 (205) (4. 0) = (P 2)(12) 820 = (P 2) 12 68. 3 L = P 2
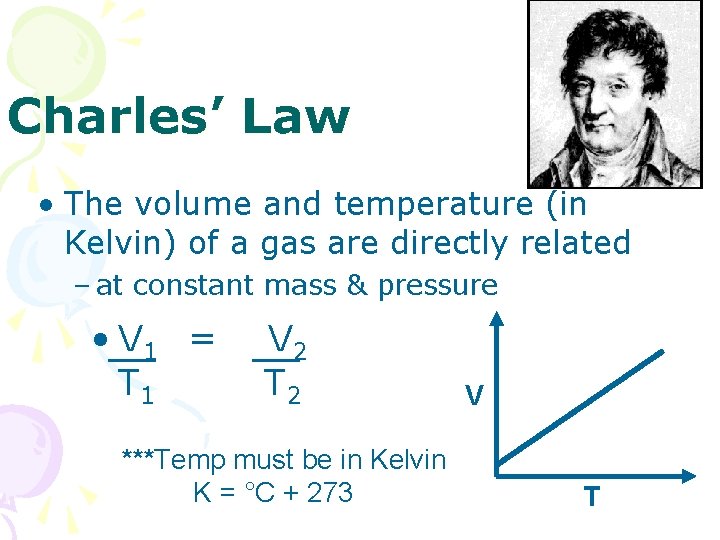
Charles’ Law • The volume and temperature (in Kelvin) of a gas are directly related – at constant mass & pressure • V 1 = T 1 V 2 T 2 ***Temp must be in Kelvin K = °C + 273 V T
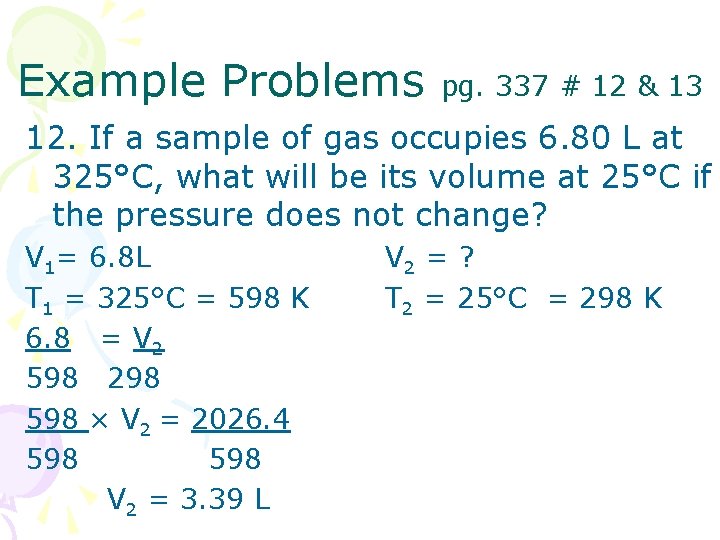
Example Problems pg. 337 # 12 & 13 12. If a sample of gas occupies 6. 80 L at 325°C, what will be its volume at 25°C if the pressure does not change? V 1= 6. 8 L T 1 = 325°C = 598 K 6. 8 = V 2 598 298 598 × V 2 = 2026. 4 598 V 2 = 3. 39 L V 2 = ? T 2 = 25°C = 298 K
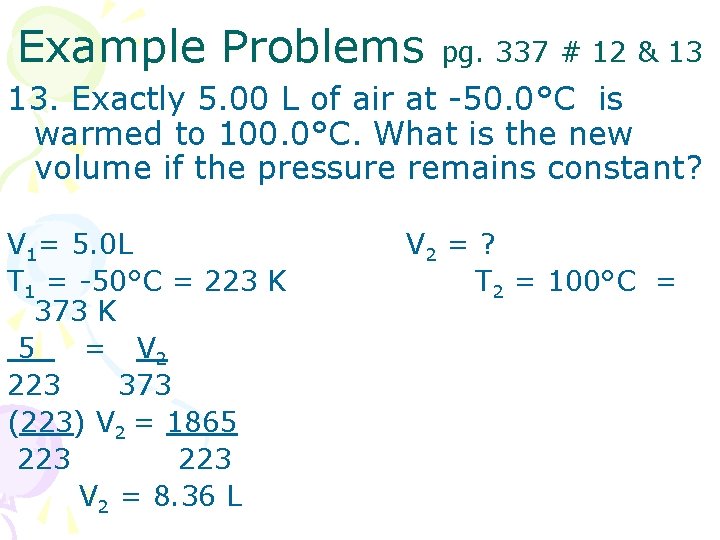
Example Problems pg. 337 # 12 & 13 13. Exactly 5. 00 L of air at -50. 0°C is warmed to 100. 0°C. What is the new volume if the pressure remains constant? V 1= 5. 0 L T 1 = -50°C = 223 K 373 K 5 = V 2 223 373 (223) V 2 = 1865 223 V 2 = 8. 36 L V 2 = ? T 2 = 100°C =
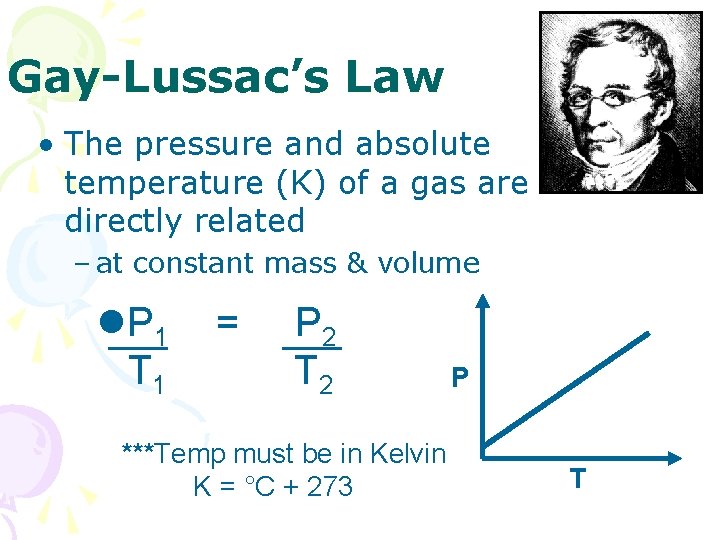
Gay-Lussac’s Law • The pressure and absolute temperature (K) of a gas are directly related – at constant mass & volume l. P 1 T 1 = P 2 T 2 ***Temp must be in Kelvin K = °C + 273 P T
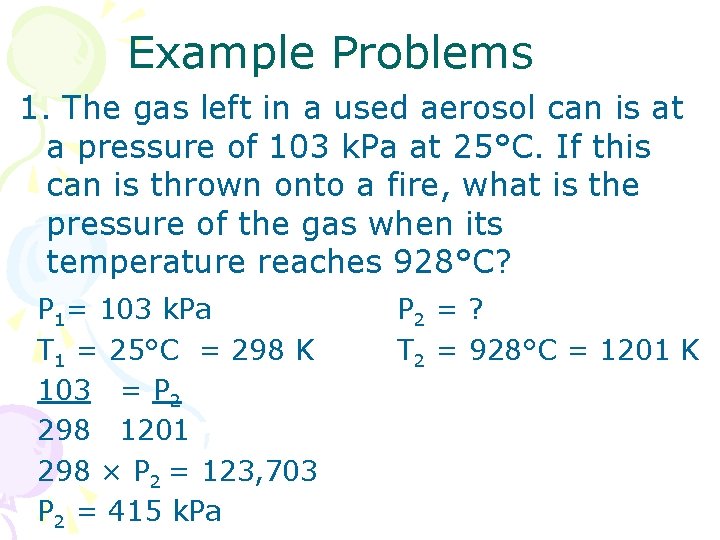
Example Problems 1. The gas left in a used aerosol can is at a pressure of 103 k. Pa at 25°C. If this can is thrown onto a fire, what is the pressure of the gas when its temperature reaches 928°C? P 1= 103 k. Pa T 1 = 25°C = 298 K 103 = P 2 298 1201 298 × P 2 = 123, 703 P 2 = 415 k. Pa P 2 = ? T 2 = 928°C = 1201 K
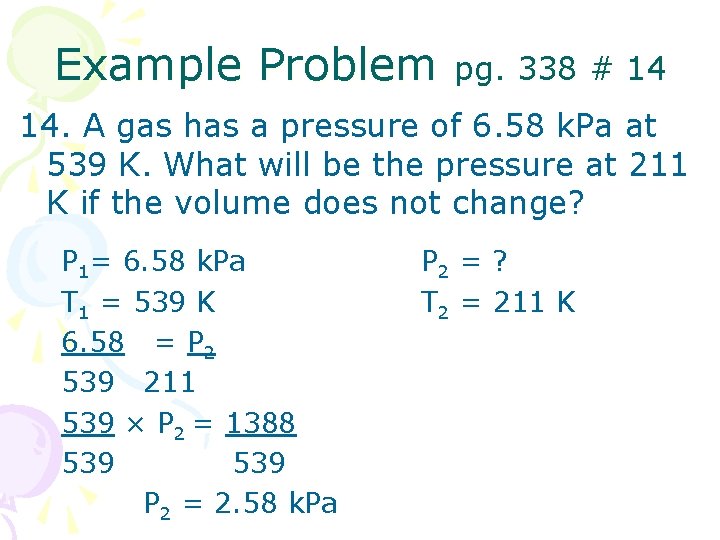
Example Problem pg. 338 # 14 14. A gas has a pressure of 6. 58 k. Pa at 539 K. What will be the pressure at 211 K if the volume does not change? P 1= 6. 58 k. Pa T 1 = 539 K 6. 58 = P 2 539 211 539 × P 2 = 1388 539 P 2 = 2. 58 k. Pa P 2 = ? T 2 = 211 K
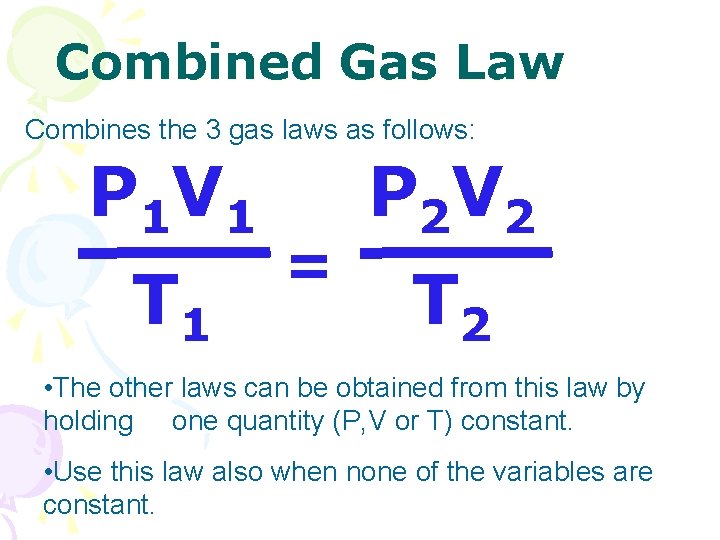
Combined Gas Law Combines the 3 gas laws as follows: P 1 V 1 T 1 = P 2 V 2 T 2 • The other laws can be obtained from this law by holding one quantity (P, V or T) constant. • Use this law also when none of the variables are constant.
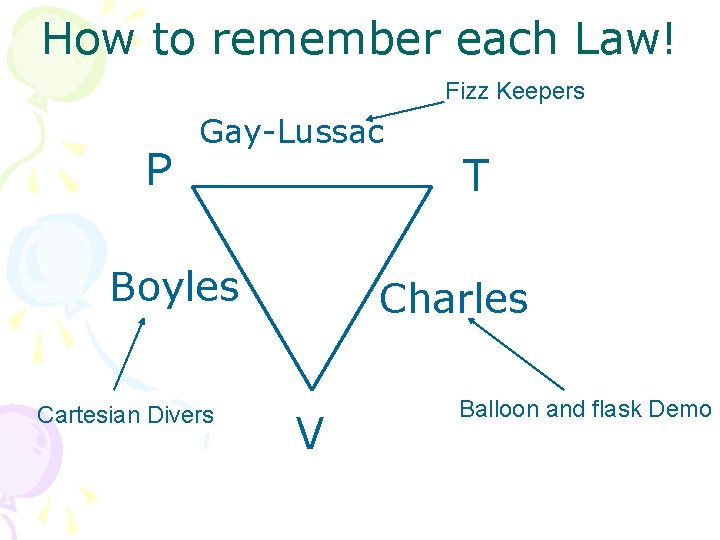
How to remember each Law! Fizz Keepers P Gay-Lussac Boyles Cartesian Divers T Charles V Balloon and flask Demo
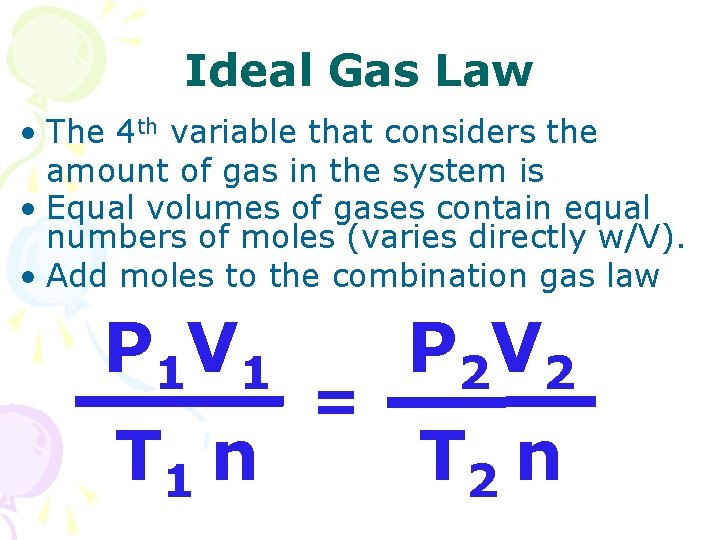
Ideal Gas Law • The 4 th variable that considers the amount of gas in the system is • Equal volumes of gases contain equal numbers of moles (varies directly w/V). • Add moles to the combination gas law P 1 V 1 T 1 n = P 2 V 2 T 2 n
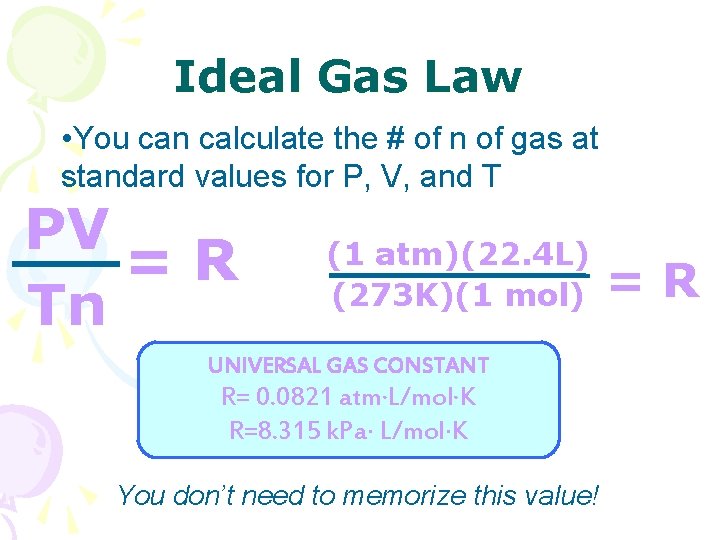
Ideal Gas Law • You can calculate the # of n of gas at standard values for P, V, and T PV =R Tn (1 atm)(22. 4 L) (273 K)(1 mol) UNIVERSAL GAS CONSTANT R= 0. 0821 atm∙L/mol∙K R=8. 315 k. Pa L/mol K You don’t need to memorize this value! =R

Ideal Gas Law PV=n. RT UNIVERSAL GAS CONSTANT R= 0. 0821 atm∙L/mol∙K R=8. 315 k. Pa L/mol K You don’t need to memorize these values!

Example Problems 1. At what temperature will 5. 00 g of Cl 2 exert a pressure of 900 mm Hg at a volume of 750 m. L? 2. Find the number of grams of CO 2 that exert a pressure of 785 mm Hg at a volume of 32. 5 L and a temperature of 32 degrees Celsius. 3. What volume will 454 g of H 2 occupy at 1. 05 atm and 25°C.
- Slides: 16