Ch 14 Gases II Ideal Gas Law and

- Slides: 21

Ch. 14 - Gases II. Ideal Gas Law and Gas Stoichiometry

Part 1 Ideal Gas Law

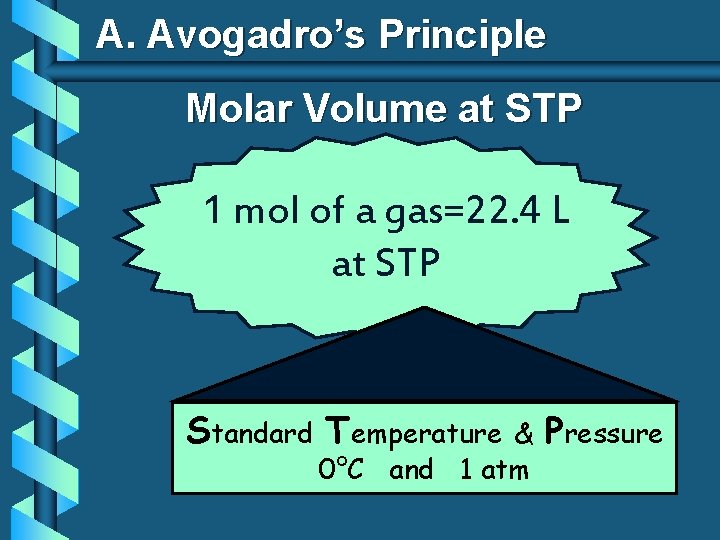
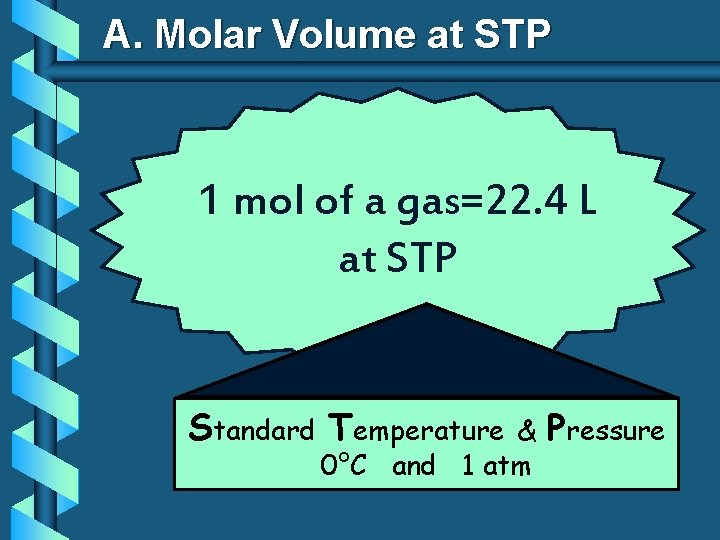
A. Avogadro’s Principle Molar Volume at STP 1 mol of a gas=22. 4 L at STP Standard Temperature & 0°C and 1 atm Pressure

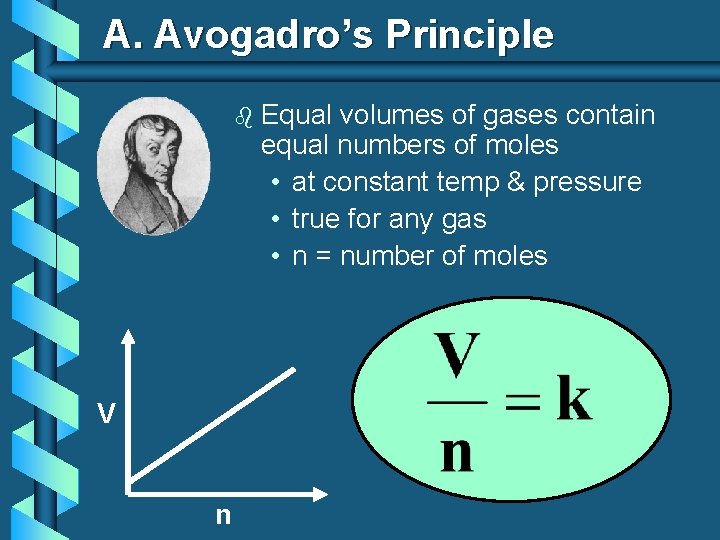
A. Avogadro’s Principle b V n Equal volumes of gases contain equal numbers of moles • at constant temp & pressure • true for any gas • n = number of moles

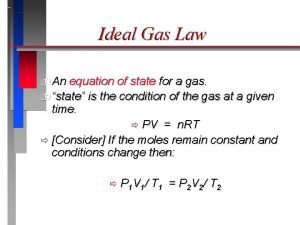
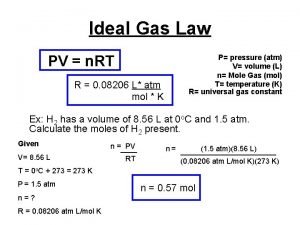
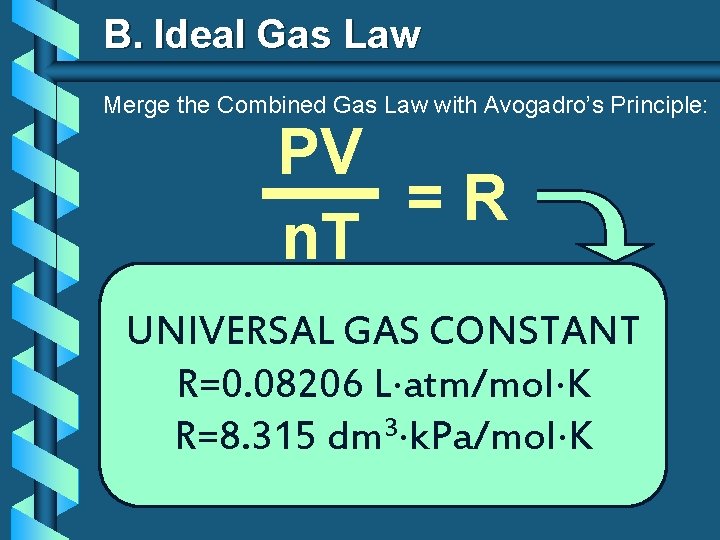
B. Ideal Gas Law Merge the Combined Gas Law with Avogadro’s Principle: PV V =R k n. T T n UNIVERSAL GAS CONSTANT R=0. 08206 L atm/mol K R=8. 315 dm 3 k. Pa/mol K

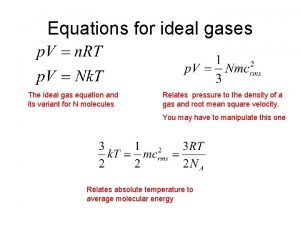
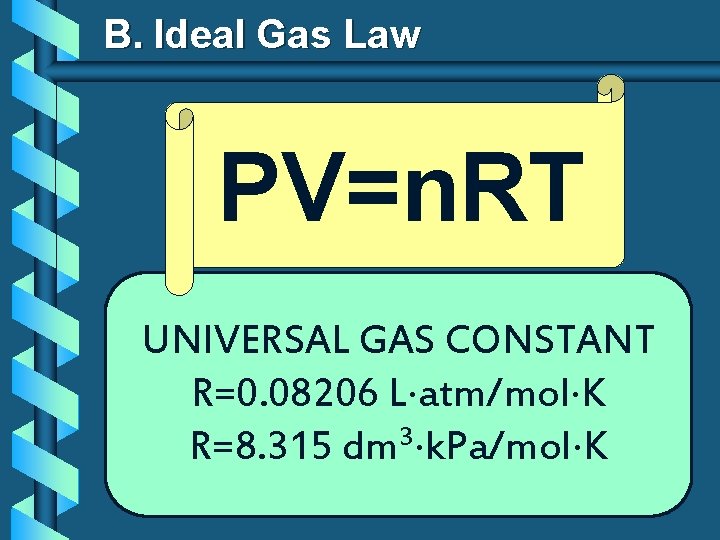
B. Ideal Gas Law PV=n. RT UNIVERSAL GAS CONSTANT R=0. 08206 L atm/mol K R=8. 315 dm 3 k. Pa/mol K

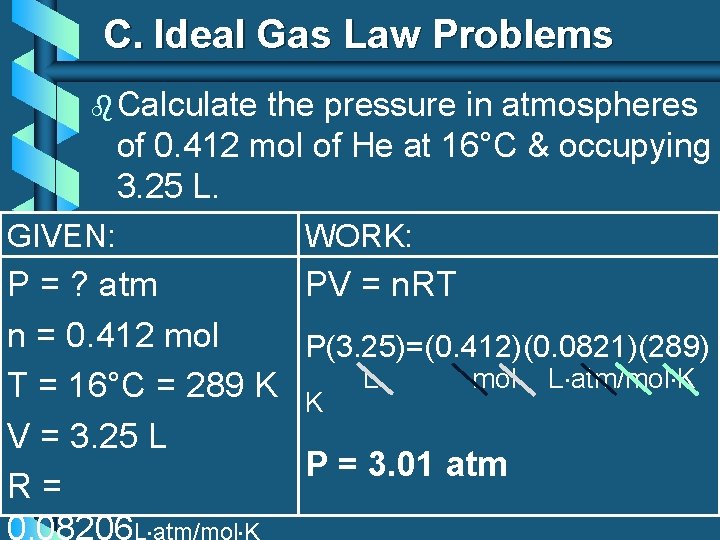
C. Ideal Gas Law Problems b Calculate the pressure in atmospheres of 0. 412 mol of He at 16°C & occupying 3. 25 L. GIVEN: WORK: P = ? atm PV = n. RT n = 0. 412 mol P(3. 25)=(0. 412)(0. 0821)(289) mol L atm/mol K T = 16°C = 289 K K L V = 3. 25 L P = 3. 01 atm R= 0. 08206 L atm/mol K

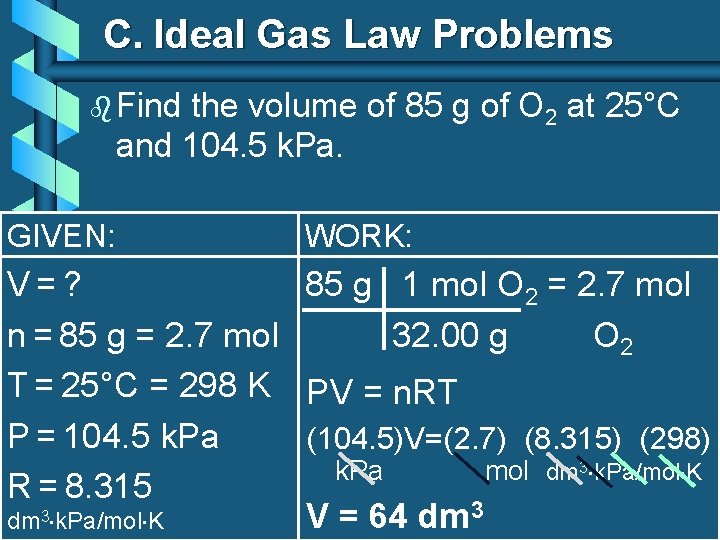
C. Ideal Gas Law Problems b Find the volume of 85 g of O 2 at 25°C and 104. 5 k. Pa. GIVEN: WORK: V=? 85 g 1 mol O 2 = 2. 7 mol n = 85 g = 2. 7 mol 32. 00 g O 2 T = 25°C = 298 K PV = n. RT P = 104. 5 k. Pa (104. 5)V=(2. 7) (8. 315) (298) k. Pa mol dm 3 k. Pa/mol K R = 8. 315 V = 64 dm 3 k. Pa/mol K

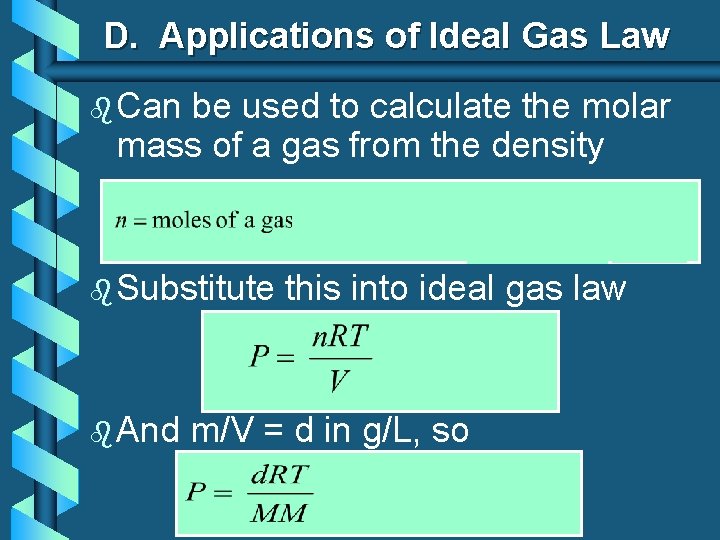
D. Applications of Ideal Gas Law b Can be used to calculate the molar mass of a gas from the density b Substitute b And this into ideal gas law m/V = d in g/L, so

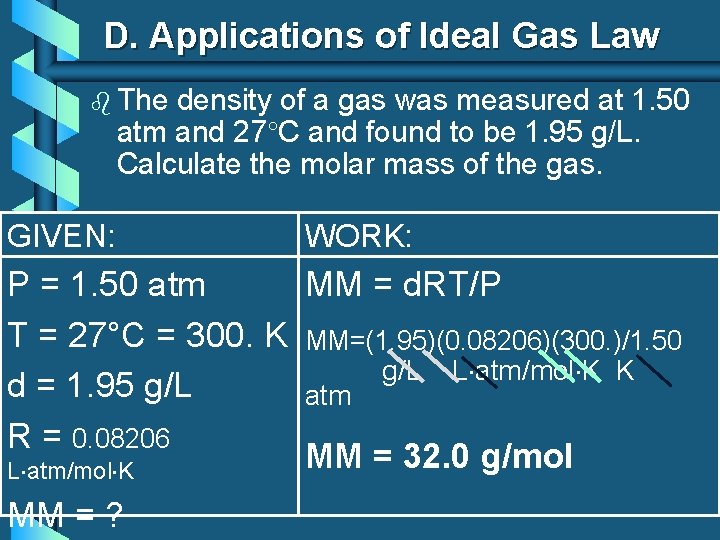
D. Applications of Ideal Gas Law b The density of a gas was measured at 1. 50 atm and 27°C and found to be 1. 95 g/L. Calculate the molar mass of the gas. GIVEN: WORK: MM = d. RT/P P = 1. 50 atm T = 27°C = 300. K MM=(1. 95)(0. 08206)(300. )/1. 50 g/L L atm/mol K K d = 1. 95 g/L atm R = 0. 08206 MM = 32. 0 g/mol L atm/mol K MM = ?

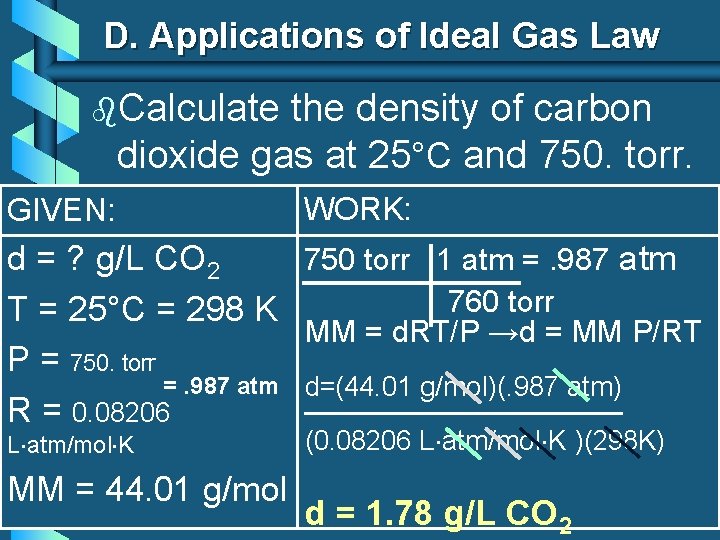
D. Applications of Ideal Gas Law b. Calculate the density of carbon dioxide gas at 25°C and 750. torr. GIVEN: WORK: 750 torr 1 atm =. 987 atm d = ? g/L CO 2 760 torr T = 25°C = 298 K MM = d. RT/P →d = MM P/RT P = 750. torr =. 987 atm d=(44. 01 g/mol)(. 987 atm) R = 0. 08206 L atm/mol K MM = 44. 01 g/mol (0. 08206 L atm/mol K )(298 K) d = 1. 78 g/L CO 2

Part 2 Gas Stoichiometry

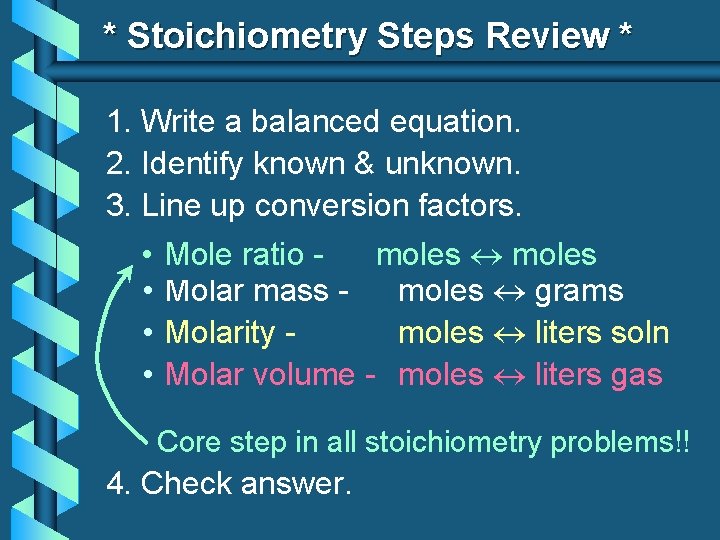
* Stoichiometry Steps Review * 1. Write a balanced equation. 2. Identify known & unknown. 3. Line up conversion factors. Mole ratio -moles • • Mole moles • Molar mass moles grams • Molarity moles liters soln • Molar volume - moles liters gas Core step in all stoichiometry problems!! 4. Check answer.

A. Molar Volume at STP 1 mol of a gas=22. 4 L at STP Standard Temperature & 0°C and 1 atm Pressure

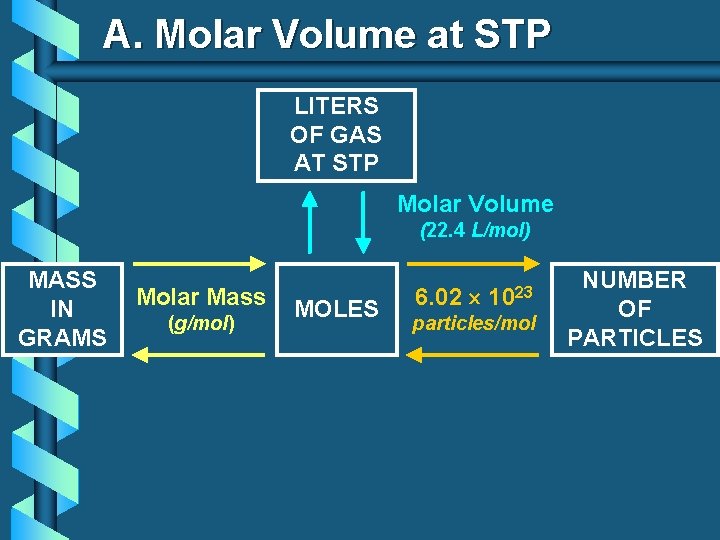
A. Molar Volume at STP LITERS OF GAS AT STP Molar Volume (22. 4 L/mol) MASS IN GRAMS Molar Mass (g/mol) MOLES 6. 02 1023 particles/mol NUMBER OF PARTICLES

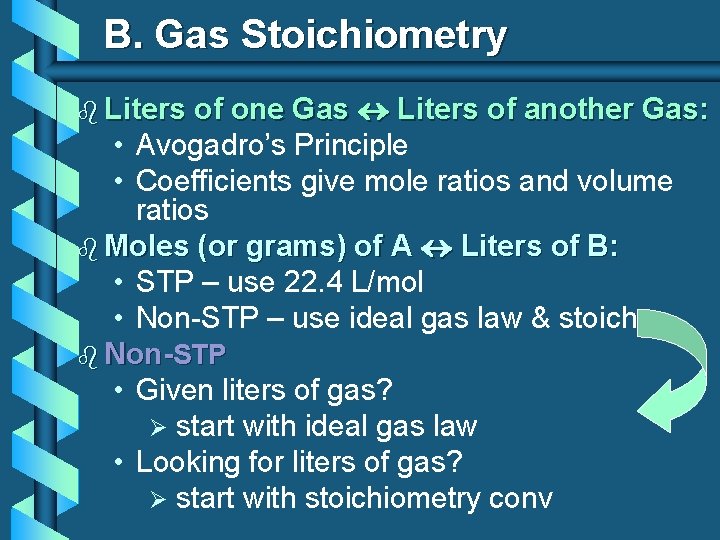
B. Gas Stoichiometry of one Gas Liters of another Gas: • Avogadro’s Principle • Coefficients give mole ratios and volume ratios b Moles (or grams) of A Liters of B: • STP – use 22. 4 L/mol • Non-STP – use ideal gas law & stoich b Non-STP • Given liters of gas? Ø start with ideal gas law • Looking for liters of gas? Ø start with stoichiometry conv b Liters

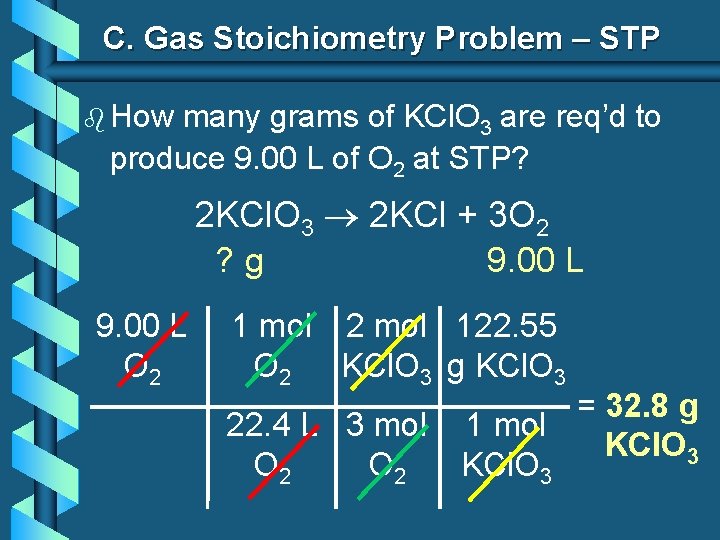
C. Gas Stoichiometry Problem – STP b How many grams of KCl. O 3 are req’d to produce 9. 00 L of O 2 at STP? 2 KCl. O 3 2 KCl + 3 O 2 ? g 9. 00 L O 2 1 mol 2 mol 122. 55 O 2 KCl. O 3 g KCl. O 3 22. 4 L 3 mol O 2 = 32. 8 g 1 mol KCl. O 3

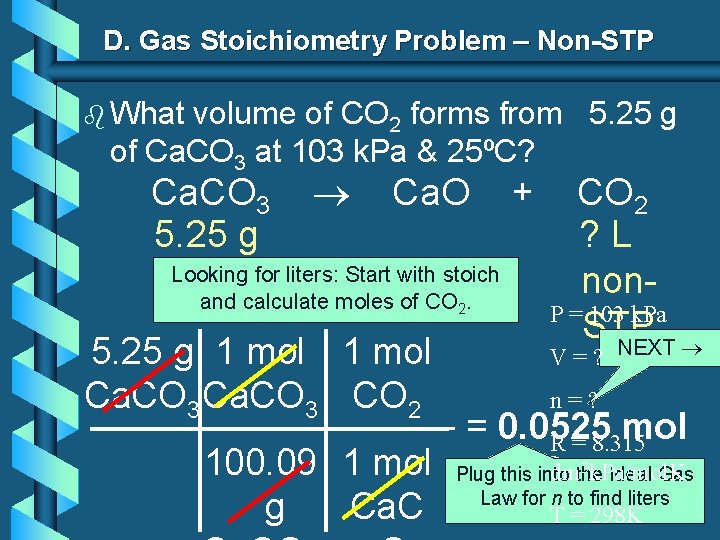
D. Gas Stoichiometry Problem – Non-STP b What volume of CO 2 forms from 5. 25 g of Ca. CO 3 at 103 k. Pa & 25ºC? Ca. CO 3 5. 25 g Ca. O Looking for liters: Start with stoich and calculate moles of CO 2. 5. 25 g 1 mol Ca. CO 3 CO 2 + CO 2 ? L non. P = 103 k. Pa STP NEXT V=? n=? = 0. 0525 mol R = 8. 315 3 k. Pa/mol. K 100. 09 1 mol Plug this dmthe into CO 2 Ideal Gas Law for n to find liters g Ca. C T = 298 K

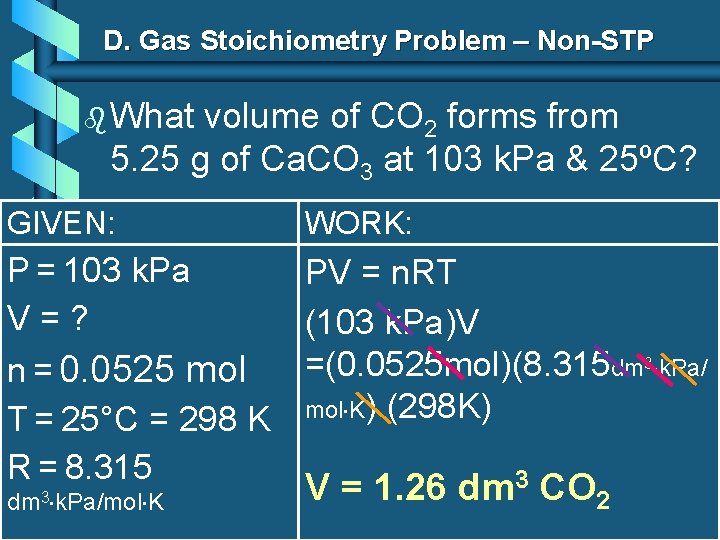
D. Gas Stoichiometry Problem – Non-STP b What volume of CO 2 forms from 5. 25 g of Ca. CO 3 at 103 k. Pa & 25ºC? GIVEN: WORK: P = 103 k. Pa PV = n. RT V=? (103 k. Pa)V =(0. 0525 mol)(8. 315 dm 3 k. Pa/ n = 0. 0525 mol T = 25°C = 298 K mol K) (298 K) R = 8. 315 3 dm 3 k. Pa/mol K V = 1. 26 dm CO 2

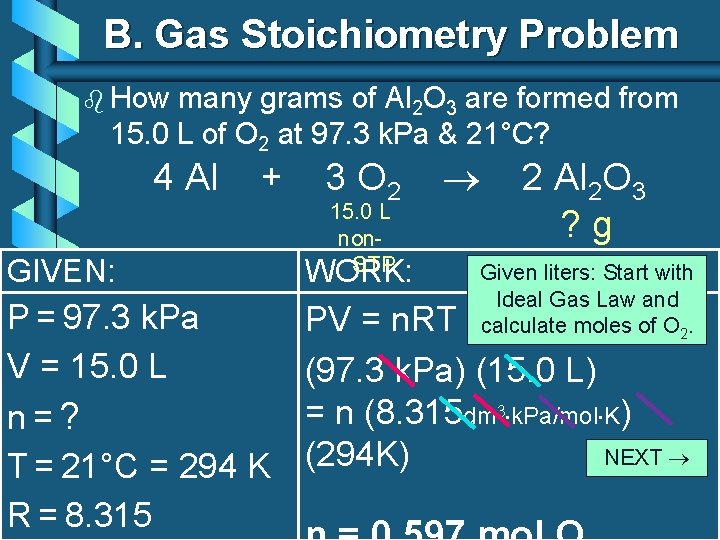
B. Gas Stoichiometry Problem b How many grams of Al 2 O 3 are formed from 15. 0 L of O 2 at 97. 3 k. Pa & 21°C? 4 Al + GIVEN: P = 97. 3 k. Pa V = 15. 0 L n=? T = 21°C = 294 K R = 8. 315 3 O 2 15. 0 L non. STP WORK: 2 Al 2 O 3 ? g Given liters: Start with Ideal Gas Law and calculate moles of O 2. PV = n. RT (97. 3 k. Pa) (15. 0 L) = n (8. 315 dm 3 k. Pa/mol K) NEXT (294 K)

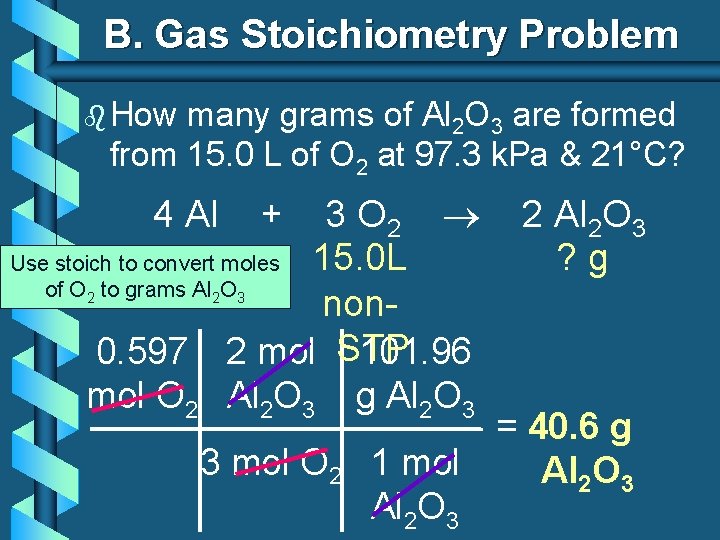
B. Gas Stoichiometry Problem b How many grams of Al 2 O 3 are formed from 15. 0 L of O 2 at 97. 3 k. Pa & 21°C? 3 O 2 Use stoich to convert moles 15. 0 L of O to grams Al O non 0. 597 2 mol STP 101. 96 mol O 2 Al 2 O 3 g Al 2 O 3 4 Al 2 2 + 2 Al 2 O 3 ? g 3 3 mol O 2 = 40. 6 g 1 mol Al 2 O 3
 Gas law
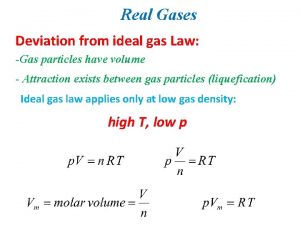
Gas law Difference between ideal gas and real gas
Difference between ideal gas and real gas Derive ideal gas equation
Derive ideal gas equation An ideal gas is an imaginary gas
An ideal gas is an imaginary gas Sutherland's law
Sutherland's law Ideal gas law with density
Ideal gas law with density Unit of pressure
Unit of pressure Combined gas law
Combined gas law Ideal gas law equation
Ideal gas law equation Which equation agrees with the ideal gas law?
Which equation agrees with the ideal gas law? Deviations from ideal gas law
Deviations from ideal gas law Ideal gas law example
Ideal gas law example Deviations from ideal gas law
Deviations from ideal gas law Ideal gas law graphs
Ideal gas law graphs Ideal gas equation
Ideal gas equation Pressure units
Pressure units Ideal gas law equation
Ideal gas law equation Ideal gas law formula
Ideal gas law formula How to find density in ideal gas law
How to find density in ideal gas law Ideal gas law to find density
Ideal gas law to find density Pzmore
Pzmore N = pv/rt
N = pv/rt