Ideal Gas Law Gas Stoichiometry Ideal Gas Law







- Slides: 20

Ideal Gas Law & Gas Stoichiometry

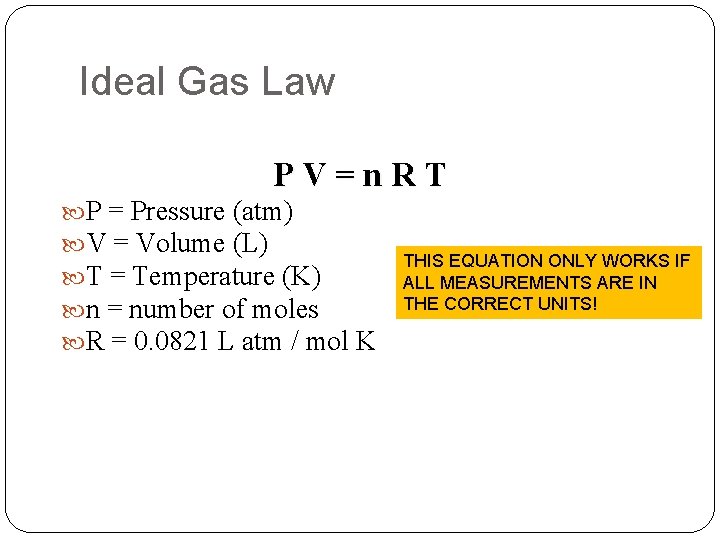
Ideal Gas Law PV=n. RT P = Pressure (atm) V = Volume (L) T = Temperature (K) n = number of moles R = 0. 0821 L atm / mol K THIS EQUATION ONLY WORKS IF ALL MEASUREMENTS ARE IN THE CORRECT UNITS!

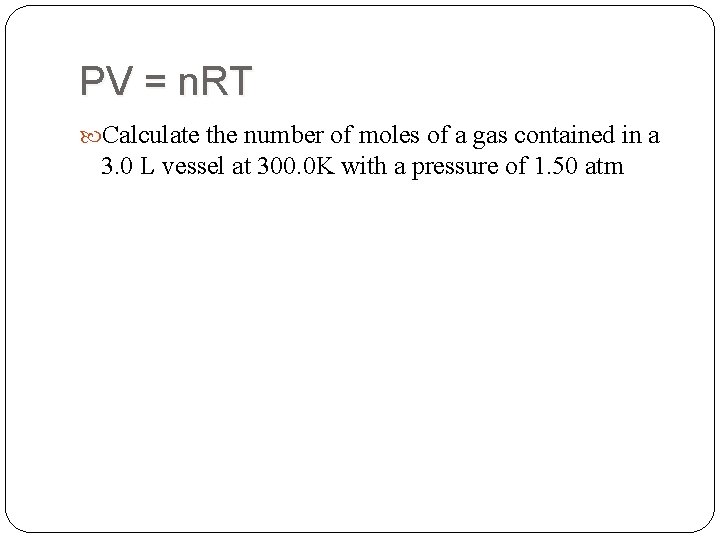
PV = n. RT Calculate the number of moles of a gas contained in a 3. 0 L vessel at 300. 0 K with a pressure of 1. 50 atm

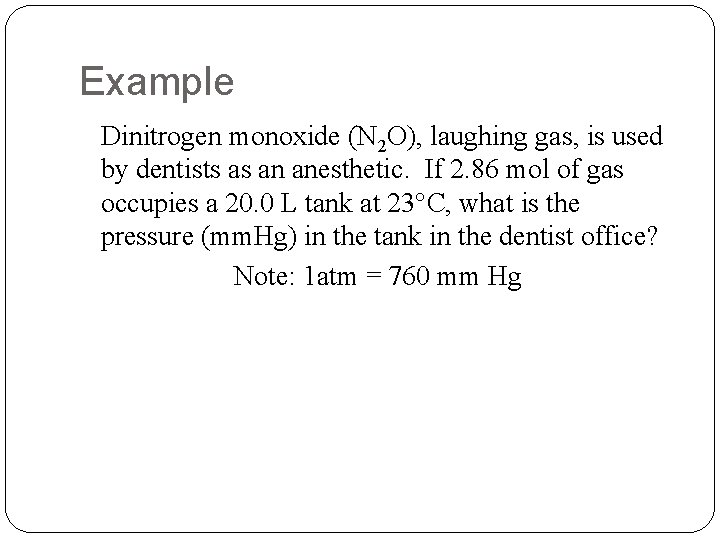
Example Dinitrogen monoxide (N 2 O), laughing gas, is used by dentists as an anesthetic. If 2. 86 mol of gas occupies a 20. 0 L tank at 23°C, what is the pressure (mm. Hg) in the tank in the dentist office? Note: 1 atm = 760 mm Hg

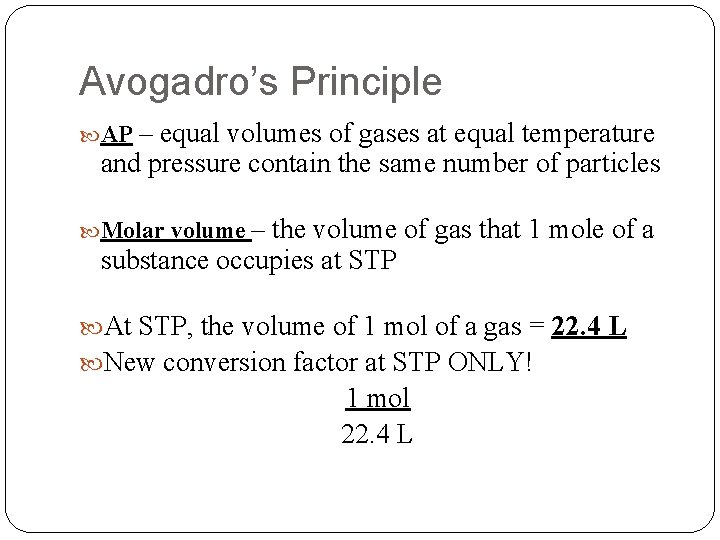
Avogadro’s Principle AP – equal volumes of gases at equal temperature and pressure contain the same number of particles Molar volume – the volume of gas that 1 mole of a substance occupies at STP At STP, the volume of 1 mol of a gas = 22. 4 L New conversion factor at STP ONLY! 1 mol 22. 4 L

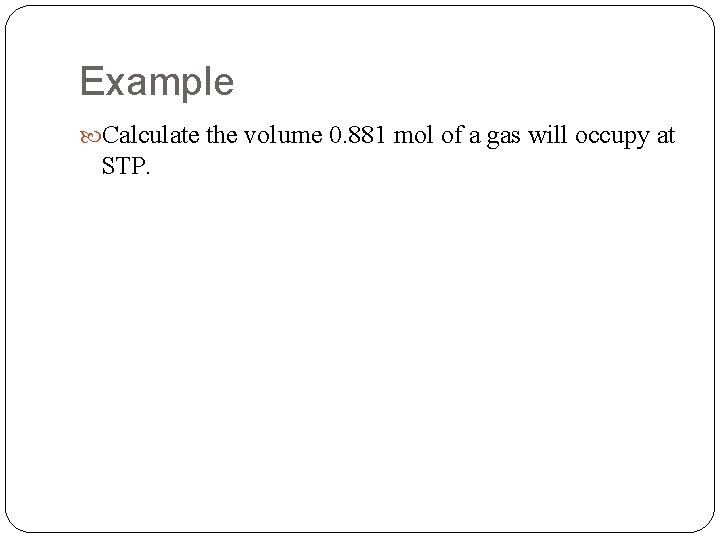
Example Calculate the volume 0. 881 mol of a gas will occupy at STP.

Example Calculate the volume that 2. 000 kg of methane would occupy at STP.

Gas Stoichiometry 2 C 4 H 10 + 13 O 2 8 CO 2 + 10 H 2 O Just like a mole to mole ratio, you can now have a liter to liter ratio

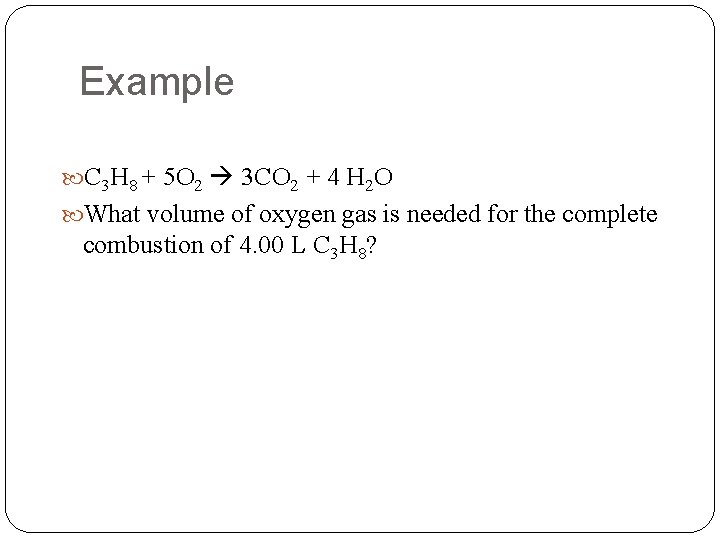
Example C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O What volume of oxygen gas is needed for the complete combustion of 4. 00 L C 3 H 8?

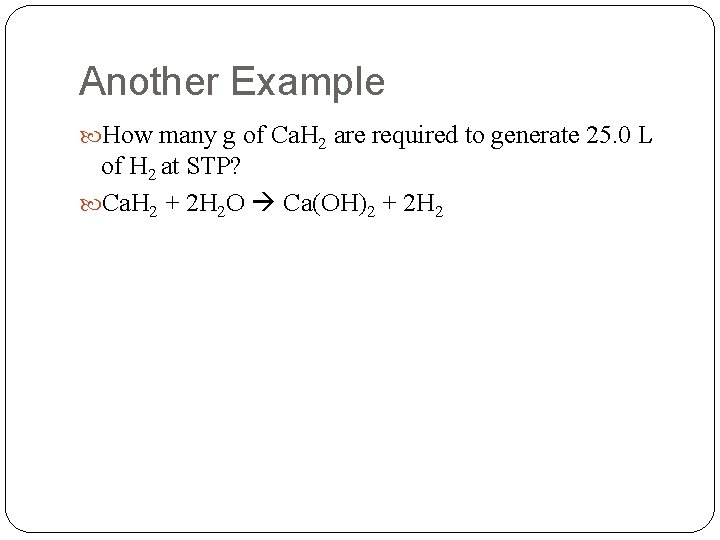
Another Example How many g of Ca. H 2 are required to generate 25. 0 L of H 2 at STP? Ca. H 2 + 2 H 2 O Ca(OH)2 + 2 H 2

Example N 2 + 3 H 2 2 NH 3 If 5. 00 L of N 2 react completely at 3. 00 atm and 298 K, how many grams of ammonia are produced? You cannot use straight stoichiometry, but you can go from L of one thing to L of another any time

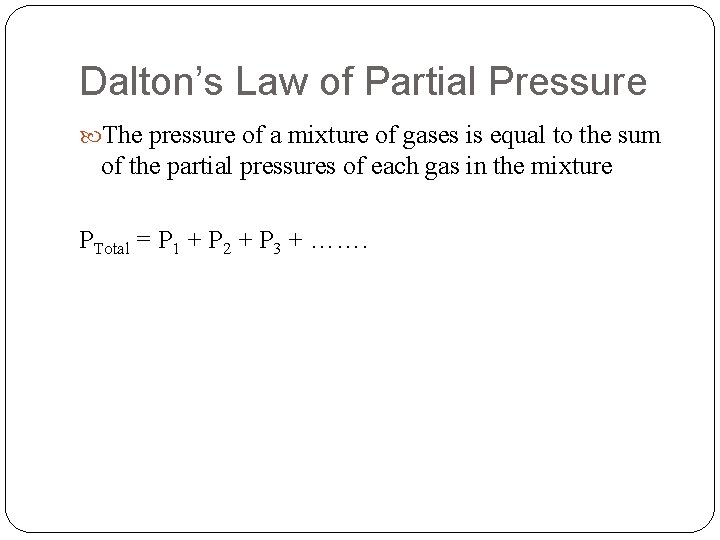
Dalton’s Law of Partial Pressure The pressure of a mixture of gases is equal to the sum of the partial pressures of each gas in the mixture PTotal = P 1 + P 2 + P 3 + …….

Example A gaseous mixture is made from 6. 00 g O 2 and 9. 00 g CH 4 in a 15. 0 L vessel at 273 K. What is the partial pressure of each gas?

Example What would be the total pressure?

Try this example What is the total pressure exerted by a mixture of 2. 00 g of hydrogen gas and 8. 00 g of nitrogen gas at 273 K in a 10. 0 L vessel?

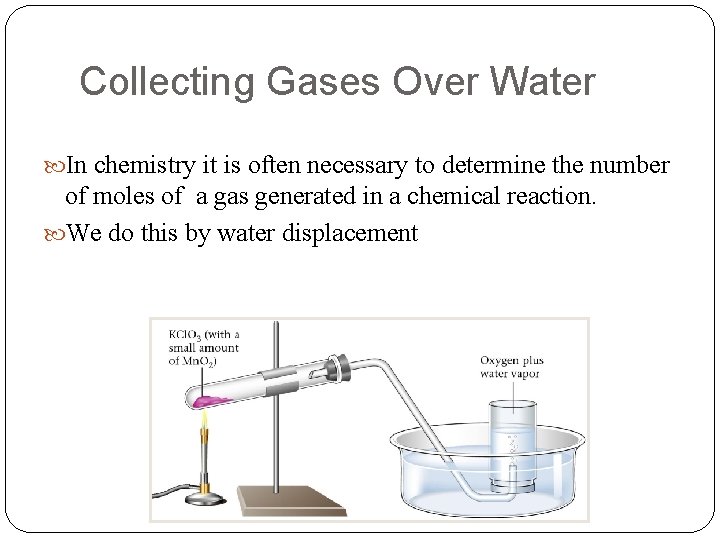
Collecting Gases Over Water In chemistry it is often necessary to determine the number of moles of a gas generated in a chemical reaction. We do this by water displacement

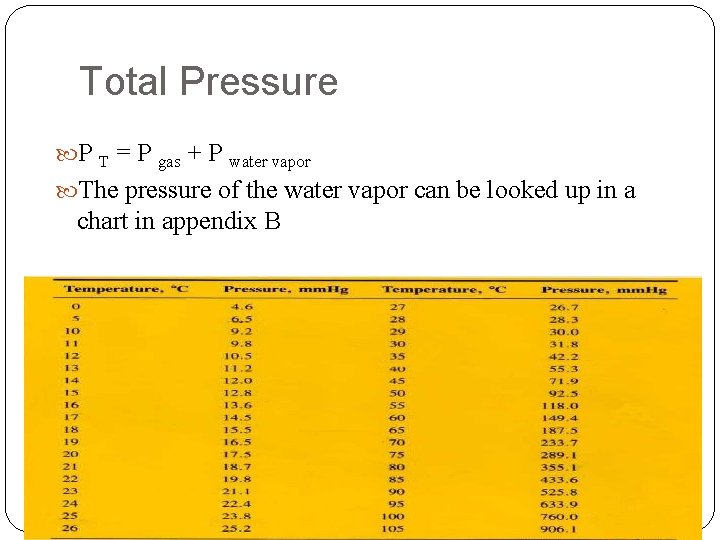
Total Pressure P T = P gas + P water vapor The pressure of the water vapor can be looked up in a chart in appendix B

Example A sample of KCl. O 3 is partially decomposed producing oxygen gas that is collected over water. The total volume of gas that is collected is 0. 250 L at 26°C and 765 torr (1 atm = 760 torr) Question #1 How many moles of oxygen were collected?

Question # 2 Calculate the number of grams of KCl. O 3 that were actually decomposed

Another Example NH 4 NO 2 N 2 + 2 H 2 O A sample of ammonium nitrite is decomposed and 511 ml of gas are collected over water at 26°C and 745 torr of total pressure. How many grams of ammonium nitrite were decomposed?