Ideal Gas Law Ideal Gases Ideal gases are




- Slides: 11

Ideal Gas Law

Ideal Gases Ideal gases are imaginary gases that perfectly fit all of the assumptions of the kinetic molecular theory (KMT). Gases consist of tiny particles that are far apart relative to their size. Collisions between gas particles and between particles and the walls of the container are elastic collisions (no KE is lost)

Ideal Gases (continued) Gas particles are in constant, random motion. There are no forces of attraction between gas particles The average kinetic energy of gas particles depends on temperature, not on the identity of the particle.

Real Gases Do Not Behave Ideally Real gases DO experience inter-molecular attractions Real gases DO have volume Real gases DO NOT have elastic collisions

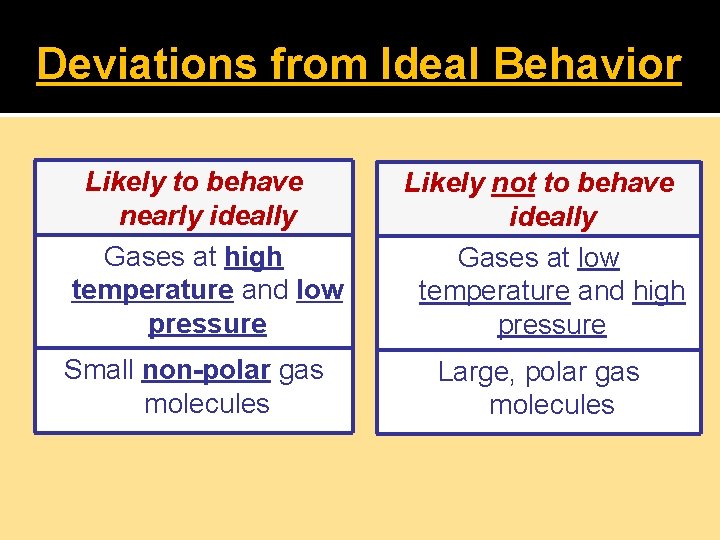
Deviations from Ideal Behavior Likely to behave nearly ideally Gases at high temperature and low pressure Small non-polar gas molecules Likely not to behave ideally Gases at low temperature and high pressure Large, polar gas molecules

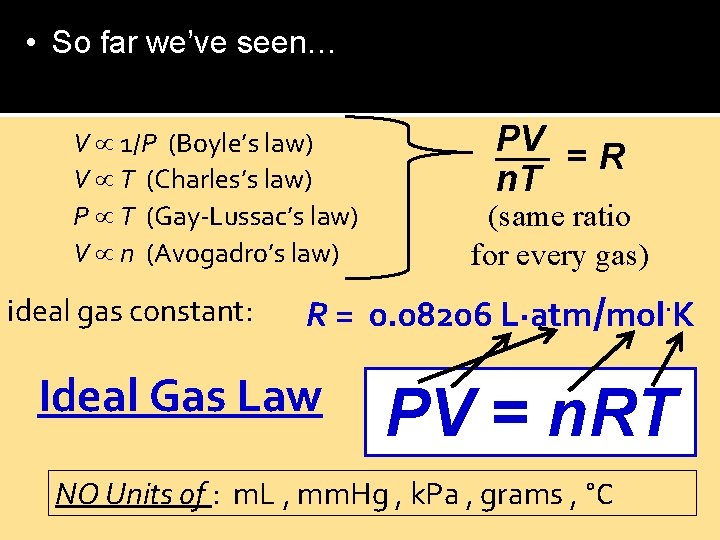
• So far we’ve seen… V 1/P (Boyle’s law) V T (Charles’s law) P T (Gay-Lussac’s law) V n (Avogadro’s law) ideal gas constant: constant PV = R n. T (same ratio for every gas) R = 0. 08206 L∙atm/mol∙K Ideal Gas Law PV = n. RT NO Units of : m. L , mm. Hg , k. Pa , grams , °C

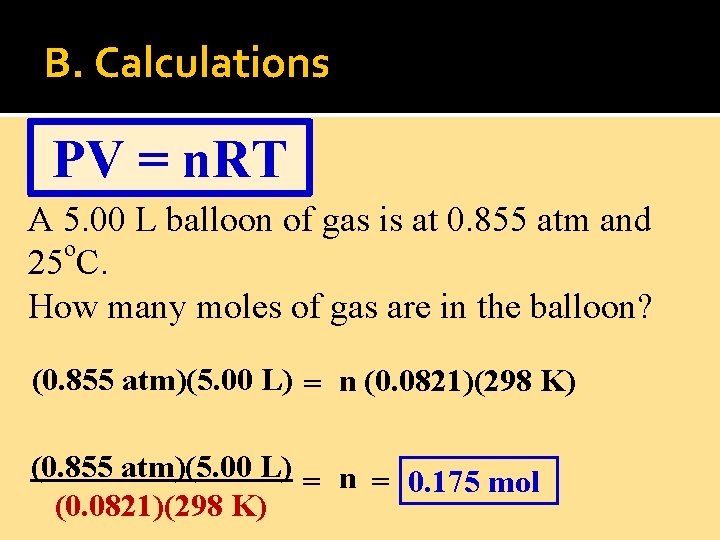
B. Calculations PV = n. RT A 5. 00 L balloon of gas is at 0. 855 atm and o 25 C. How many moles of gas are in the balloon?

B. Calculations PV = n. RT A 5. 00 L balloon of gas is at 0. 855 atm and o 25 C. How many moles of gas are in the balloon? (0. 855 atm)(5. 00 L) = n (0. 0821)(298 K) (0. 855 atm)(5. 00 L) = n = 0. 175 mol (0. 0821)(298 K)

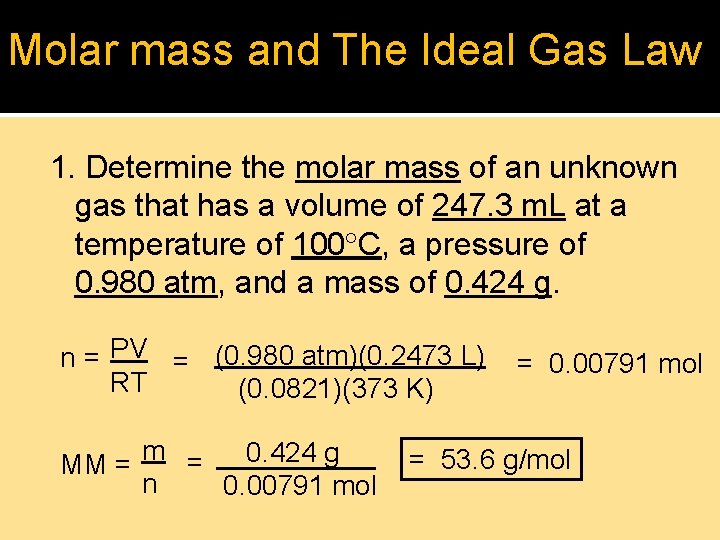
Molar mass and The Ideal Gas Law 1. Determine the molar mass of an unknown gas that has a volume of 247. 3 m. L at a temperature of 100 C, a pressure of 0. 980 atm, and a mass of 0. 424 g. n = PV = (0. 980 atm)(0. 2473 L) RT (0. 0821)(373 K) m = 0. 424 g MM = n 0. 00791 mol = 53. 6 g/mol

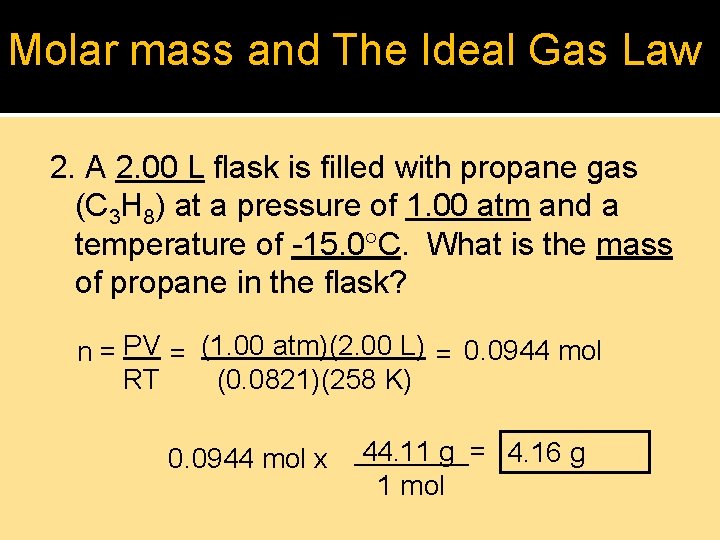
Molar mass and The Ideal Gas Law 2. A 2. 00 L flask is filled with propane gas (C 3 H 8) at a pressure of 1. 00 atm and a temperature of -15. 0 C. What is the mass of propane in the flask? n = PV = (1. 00 atm)(2. 00 L) = 0. 0944 mol RT (0. 0821)(258 K) 0. 0944 mol x 44. 11 g = 4. 16 g 1 mol

Quick Quiz! Find the volume of a gas if 2. 95 mol has a pressure of 0. 756 atm at a temperature of 52. 0°C. A) 22. 4 L B) 16. 7 L C) 104 L D) 59. 5 L